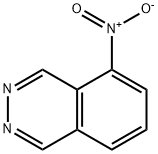
5-NITROPHTHALAZINE synthesis
- Product Name:5-NITROPHTHALAZINE
- CAS Number:89898-86-2
- Molecular formula:C8H5N3O2
- Molecular Weight:175.14
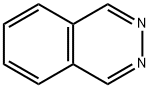
253-52-1

89898-86-2
The general procedure for the synthesis of 5-nitrophthalazine from 2,3-diazanaphthalene was as follows: 3.0 g (23.3 mmol) of 2,3-diazanaphthalene was dissolved in 20 mL of concentrated sulfuric acid and heated up to 100°C. The reaction was carried out in the following manner. Within 1 hour, 18.8 g (186 mmol) of potassium nitrate was added to the reaction system in batches. The reaction temperature was maintained at 100 °C for 72 hours. Upon completion of the reaction, the reaction solution was cooled to room temperature, slowly poured into ice water and neutralized to neutrality with ammonium hydroxide solution, at which time a yellow-brown precipitate was generated. The precipitate was collected by filtration and dried to give 2.3 g (56% yield) of 5-nitrophthalazine intermediate as a light yellow solid. The structure of the product was confirmed by 1H NMR (400 MHz, DMSO-d6) and 13C NMR (100.17 MHz, DMSO-d6) as follows: 1H NMR δ: 10.2 (s, 1H), 9.98 (s, 1H), 8.84 (d, J = 7.4 Hz, 1H), 8.59 (d, J = 7.6 Hz. 1H), 8.20 (dd, J = 7.4,14.9 Hz, 1H); 13C NMR δ: 152.1,146.3,141.0,133.2,131.8,130.0,127.4,118.7.

253-52-1
214 suppliers
$7.00/1g

89898-86-2
63 suppliers
$45.00/50mg
Yield:89898-86-2 64.3%
Reaction Conditions:
with sulfuric acid;potassium nitrate at 0 - 80;
References:
Shen, De-Qing;Wu, Zu-Ping;Wu, Xi-Wei;An, Zeng-Yun;Bu, Xiang-Zhang;Gu, Lian-Quan;Huang, Zhi-Shu;An, Lin-Kun [European Journal of Medicinal Chemistry,2010,vol. 45,# 9,p. 3938 - 3942] Location in patent:experimental part
484-23-1
53 suppliers
inquiry

89898-86-2
63 suppliers
$45.00/50mg
91-15-6
273 suppliers
$13.00/50g

89898-86-2
63 suppliers
$45.00/50mg

253-52-1
214 suppliers
$7.00/1g
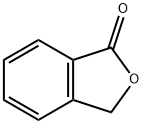
87-41-2
484 suppliers
$6.00/10g
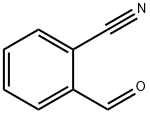
7468-67-9
251 suppliers
$13.00/5g

89898-86-2
63 suppliers
$45.00/50mg

91-15-6
273 suppliers
$13.00/50g