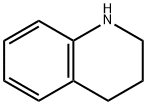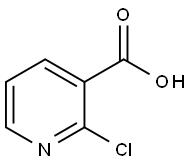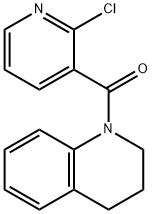
(2-Chloro-3-pyridinyl)[3,4-dihydro-1(2H)-quinolinyl]methanone synthesis
- Product Name:(2-Chloro-3-pyridinyl)[3,4-dihydro-1(2H)-quinolinyl]methanone
- CAS Number:545340-46-3
- Molecular formula:C15H13ClN2O
- Molecular Weight:272.73
Yield:545340-46-3 88%
Reaction Conditions:
with 2-chloro-1-methyl-pyridinium iodide;triethylamine in dichloromethane at 20; for 2 h;
Steps:
6.1
Step 1: (2-Chloro-pyridin-3-yl)-(3,4-dihydro-2H-quinolin-1-yl)-methanone To a solution of 2-chloro-nicotinic acid (1.0 g, 6.35 mmol, 1.0 equiv; [CAS RN 2942-59-8]) in dichloromethane (40 mL) was added 1,2,3,4-tetrahydro-quinoline (0.93 g, 0.88 mL, 6.98 mmol, 1.1 equiv; [CAS RN 635-46-1]), triethylamine (1.28 g, 1.77 mL, 12.69 mmol, 2.0 equiv; [CAS RN 121-44-8]) and 2-chloro-1-methylpyridinium iodide (1.96 g, 6.98 mmol, 1.1 equiv; [CAS RN 14338-32-0]). The reaction mixture was stirred at rt for 2 h. To the residue was added a sat. solution of NaHCO3 (100 mL) and the solution extracted with dichloromethane (3*50 mL). The combined organic phases were dried over Na2SO4 and the product purified by silica column chromatography using a MPLC system (CombiFlash Companion, Isco Inc.) eluding with a gradient of heptane/ethyl acetate affording 1.52 g (88%) of the title compound as a light yellow solid. 1H NMR (400 MHz, CDCl3): δ2.07 (quint, J=6.6 Hz, 2H), 2.86 (t, J=6.6 Hz, 2H), 3.90 (t, J=6.4 Hz, 2H), 6.64 (br s, 1H), 6.93 (br t, J=4.0 Hz, 1H), 7.04 (br d, J=4.0 Hz, 1H), 7.08 (t, J=7.6 Hz, 1H), 7.19 (d, J=7.6 Hz, 1H), 7.30 (s, 1H), 8.31 (d, J=7.6 Hz, 1H). MS (ISP): 273.1 [M+H]+.
References:
US2010/105906,2010,A1 Location in patent:Page/Page column 23