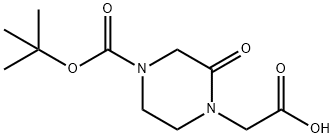
4-CARBOXYMETHYL-3-OXO-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER synthesis
- Product Name:4-CARBOXYMETHYL-3-OXO-PIPERAZINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
- CAS Number:549506-47-0
- Molecular formula:C11H18N2O5
- Molecular Weight:258.27
![1-Piperazineacetic acid, 4-[(1,1-dimethylethoxy)carbonyl]-2-oxo-, ethyl ester](/CAS/20210305/GIF/1198287-13-6.gif)
1198287-13-6
0 suppliers
inquiry

549506-47-0
69 suppliers
$84.00/250MG
Yield:549506-47-0 85%
Reaction Conditions:
Stage #1: C13H22N2O5with water;sodium hydroxide in methanol; for 2.5 h;
Stage #2: in water; pH=2;Acidic conditions;
Steps:
1.A
A. Synthesis of 2-(4-tert-butox carbonyl)-2-oxopiperazin-l-yl)acetic acid[0099] Under nitrogen and at room temperature, to a solution of 1 -Boc-3- oxopiperazine (10.4 g, 51.9 mmol) in anhydrous DMF (100 mL) was added NaH (60% in mineral oil, 2.50 g. 62.3 mmol) portionwise. After addition, the mixture was stirred at room temperature for 30 min and then at 65 °C for 1 h. After the mixture was cooled to room temperature, ethyl chloroacetate (7.63 g, 62.3 mmol) was added. The reaction mixture was stirred at room temperature for 30 min and at 65 °C for 2 h. Water (5 mL) was added, and the solution concentrated. Brine (100 mL) was added, and the reaction mixture was extracted with EtOAc (5 x 100 mL). The combined extracts were washed with water (100 mL) and dried over anhydrous Na2S0 . After filtration, the filtrate was concentrated by rotary evaporation, and the residue was purified by flash chromatography on silica gel (EtOAc/hexances: 1/1 v/v) to give the desired product as a white solid (13 g, 87%). [00100] The solid was dissolved in methanol (50 mL) and treated with aqueous NaOH (2.5 N, 50 mL) for 2.5 h. The methanol was removed, and the residual aqueous solution was repeatedly acidified (carefully to pH = 2 each time) and then extracted with EtOAc (10 x 100 mL). The combined extracts were dried over anhydrous Na2S04 and concentrated to afford the acid as a white solid (1 1.2 g, 85%).
References:
WO2011/26241,2011,A1 Location in patent:Page/Page column 32-33
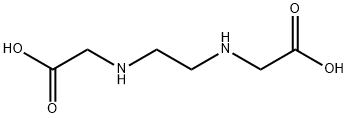
5657-17-0
111 suppliers
$34.00/1g

549506-47-0
69 suppliers
$84.00/250MG

24424-99-5
861 suppliers
$13.50/25G
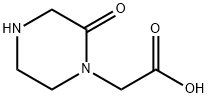
24860-46-6
56 suppliers
$73.00/50mg

549506-47-0
69 suppliers
$84.00/250MG
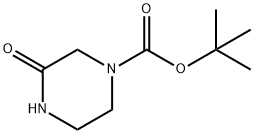
76003-29-7
243 suppliers
$5.00/5g

549506-47-0
69 suppliers
$84.00/250MG