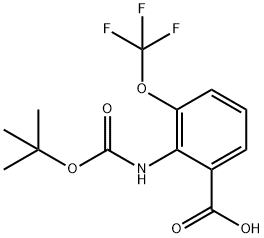
2-[(tert-butoxycarbonyl)amino]-3-(trifluoromethoxy)benzoic acid synthesis
- Product Name:2-[(tert-butoxycarbonyl)amino]-3-(trifluoromethoxy)benzoic acid
- CAS Number:561304-40-3
- Molecular formula:C13H14F3NO5
- Molecular Weight:321.25

124-38-9
122 suppliers
$214.00/14L
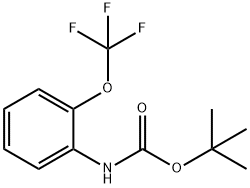
561304-39-0
20 suppliers
inquiry
![2-[(tert-butoxycarbonyl)amino]-3-(trifluoromethoxy)benzoic acid](/CAS/GIF/561304-40-3.gif)
561304-40-3
10 suppliers
$102.50/250mgs:
Yield:561304-40-3 79%
Reaction Conditions:
Stage #1: tert-butyl N-[2-(trifluoromethoxy)phenyl]carbamatewith tert.-butyl lithium in tetrahydrofuran;pentane at -78 - -50; for 1 h;
Stage #2: carbon dioxide in tetrahydrofuran;pentane;
Stage #3: with hydrogenchloride in water; pH=1;
Steps:
A
Method A: Trifluoroethyl Ester Synthesis and Cyclization; 2-[(tert-Butoxycarbonyl)amino]-3-(trifluoromethoxy)benzoic acid (117); A solution of tert-butyl [2-(trifluoromethoxy)phenyl]carbamate (139 g, 0.50 mol) in dry THF (900 mL) was cooled to -78° C. A tert-BuLi solution (1.6 M in pentane, 800 mL, 1.28 mol) was added drop-wise. After completion of the addition, the mixture was stirred at -50° C. for 1 h. The clear solution was added to solid carbon dioxide, and the mixture was left overnight. Water (900 mL) was added and the layers were separated. The aqueous layer was extracted with Et2O (500 mL) followed by acidification to pH 1 with aqueous 1 N HCl. The mixture was extracted with Et2O (2×500 mL), and the combined extracts were dried over Na2SO4, filtered and concentrated in vacuo. Trituration with pentane yielded the title compound as a white solid (128 g, 79%). 1H NMR (300 MHz, CDCl3) δ 1.51 (s, 9H), 7.26 (dd, J=8.1, 8.1 Hz, 1H), 7.47-7.53 (m, 1H), 7.74 (br s, 1H), 7.93 (dd, J=7.9, 1.5 Hz, 1H).
References:
US2010/324043,2010,A1 Location in patent:Page/Page column 73
212696-37-2
30 suppliers
$77.00/25mg
![2-[(tert-butoxycarbonyl)amino]-3-(trifluoromethoxy)benzoic acid](/CAS/GIF/561304-40-3.gif)
561304-40-3
10 suppliers
$102.50/250mgs:

24424-99-5
815 suppliers
$13.50/25G
![2-[(tert-butoxycarbonyl)amino]-3-(trifluoromethoxy)benzoic acid](/CAS/GIF/561304-40-3.gif)
561304-40-3
10 suppliers
$102.50/250mgs:
1535-75-7
319 suppliers
$5.00/5g
![2-[(tert-butoxycarbonyl)amino]-3-(trifluoromethoxy)benzoic acid](/CAS/GIF/561304-40-3.gif)
561304-40-3
10 suppliers
$102.50/250mgs: