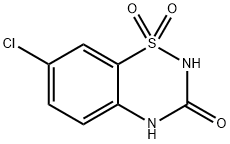
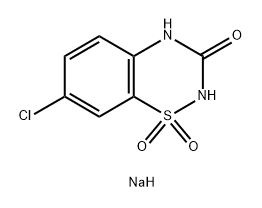
7-chloro-2H-benzo[e][1,2,4]thiadiazin-3(4H)-one 1,1-dioxide synthesis
- Product Name:7-chloro-2H-benzo[e][1,2,4]thiadiazin-3(4H)-one 1,1-dioxide
- CAS Number:5800-59-9
- Molecular formula:C7H5ClN2O3S
- Molecular Weight:232.64
Yield:5800-59-9 80%
Reaction Conditions:
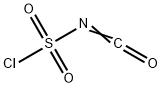
Stage #1: isocyanate de chlorosulfonyle;4-chloro-aniline in nitromethane at -10; for 0.25 h;Inert atmosphere;
Stage #2: with Aluminum Chloride in nitromethane; for 1 h;Inert atmosphere;Reflux;
Steps:
7-Chloro-3-oxo-3,4-dihydro-2H-1,2,4-benzothiadiazine1,1-dioxide(2e): General method for the synthesis of compounds 2a-2e
General procedure: A solution of chlorosulfonyl isocyanate (3.3 mL, 37.6 mmol) in nitromethane (50 mL) was mixed in a closed dried vessel under nitrogenatmosphere and cooled to -10 C (ice and salt bath). The 4-chloroaniline(1e. 4 g, 31.4 mmol) was added dropwise. The contents were vigorouslystirred for 15 mins followed by the addition of anhydrous aluminumchloride (5.44 g, 40.8 mmol) and the mixture was refluxed for 1 h. Thehot solution was poured onto ice (200 g) and stirred for an additional 30mins until all ice is melted and the resulting precipitate was collected byfiltration and washed with water. The crude solid was treated with anaqueous solution of sodium bicarbonate (5 g/100 mL) followed byheating until the solid precipitate was dissolved. The solution wastreated with charcoal and was filtered, the filtrate solution was adjustedto pH 1 using 12 N HCl. The resulting pure white precipitate was filtered,washed with water, and air dried (5.8 g, 80% yield): 1H NMR (400 MHz,DMSO-d6): δ = 7.29 (d, J = 7.7 Hz, 1H), 7.70 (d, J = 7.7 Hz, 1H), 7.83 (s,1H), 11.45 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δ = 119.6, 122.1,124.0, 127.6, 134.2, 134.5, 151.5. HRMS (ESI): m/z calcd forC7H5ClN2O3S [M + Na]+: 254.9607, found: 254.9648.
References:
Huwaimel, Bader I.;Jonnalagadda, Sravan;Jonnalagadda, Shirisha;Zahra, Fatema T.;Nocentini, Alessio;Supuran, Claudiu T.;Mikelis, Constantinos M.;Trippier, Paul C. [Bioorganic and Medicinal Chemistry,2022,vol. 67,art. no. 116805]
13338-00-6
13 suppliers
inquiry
![7-chloro-2H-benzo[e][1,2,4]thiadiazin-3(4H)-one 1,1-dioxide](/CAS/20180527/GIF/5800-59-9.gif)
5800-59-9
10 suppliers
inquiry
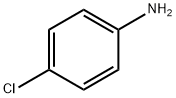
106-47-8
487 suppliers
$5.00/5G
![7-chloro-2H-benzo[e][1,2,4]thiadiazin-3(4H)-one 1,1-dioxide](/CAS/20180527/GIF/5800-59-9.gif)
5800-59-9
10 suppliers
inquiry
6124-50-1
0 suppliers
inquiry
![7-chloro-2H-benzo[e][1,2,4]thiadiazin-3(4H)-one 1,1-dioxide](/CAS/20180527/GIF/5800-59-9.gif)
5800-59-9
10 suppliers
inquiry