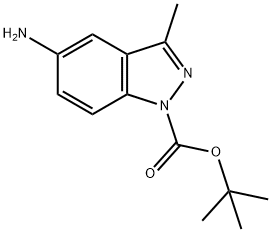
1H-Indazole-1-carboxylic acid, 5-aMino-3-Methyl-, 1,1-diMethylethyl ester synthesis
- Product Name:1H-Indazole-1-carboxylic acid, 5-aMino-3-Methyl-, 1,1-diMethylethyl ester
- CAS Number:599183-32-1
- Molecular formula:C13H17N3O2
- Molecular Weight:247.29
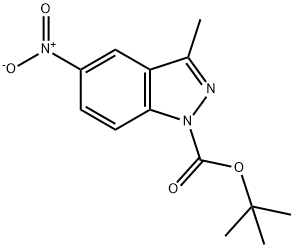
599183-33-2
19 suppliers
$376.00/500mg

599183-32-1
21 suppliers
$335.00/0.5g
Yield:599183-32-1 93%
Reaction Conditions:
with 10% Pd/C;hydrogen in methanol at 20;
Steps:
4.1.3 General procedure for the reduction of nitro groups to amine groups (Procedure C)
General procedure: A nitro compound was dissolved in MeOH, and then 10% Pd/C was added. The mixture was stirred at room temperature under hydrogen gas until the initial material was consumed. The crude mixture was filtered through Celite, washed with MeOH, and then concentrated. The product was used for the following step without further purification or was purified by silica gel column chromatography if necessary.
References:
Van Manh, Nguyen;Hoang, Van-Hai;Ngo, Van T.H.;Kang, Soosung;Jeong, Jin Ju;Ha, Hee-Jin;Kim, Hee;Kim, Young-Ho;Ann, Jihyae;Lee, Jeewoo [European Journal of Medicinal Chemistry,2022,vol. 244,art. no. 114837] Location in patent:supporting information
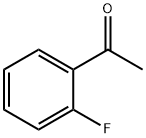
445-27-2
441 suppliers
$9.00/25g

599183-32-1
21 suppliers
$335.00/0.5g
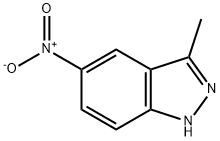
40621-84-9
123 suppliers
$19.00/100mg

599183-32-1
21 suppliers
$335.00/0.5g
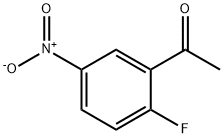
79110-05-7
166 suppliers
$20.00/5g

599183-32-1
21 suppliers
$335.00/0.5g