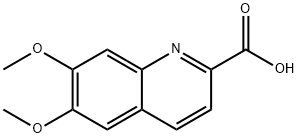
6,7-Dimethoxy-quinoline-2-carboxylic acid synthesis
- Product Name:6,7-Dimethoxy-quinoline-2-carboxylic acid
- CAS Number:122234-49-5
- Molecular formula:C12H11NO4
- Molecular Weight:233.22
617-35-6
564 suppliers
$5.00/10g
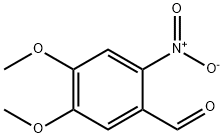
20357-25-9
282 suppliers
$5.00/25mg

122234-49-5
4 suppliers
inquiry
Yield:122234-49-5 82.6%
Reaction Conditions:
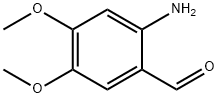
Stage #1: 6-nitroveratraldehydewith 5%-palladium/activated carbon;hydrogen in tetrahydrofuran at 25 - 30; under 750.075 - 1500.15 Torr; for 4 h;Autoclave;
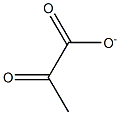
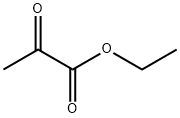
Stage #2: 2-oxo-propionic acid ethyl esterwith sodium ethanolate in water at 60 - 65; for 6 h;Solvent;Reagent/catalyst;Temperature;Pressure;
Steps:
1 Example 1 Preparation of 6,7-dimethoxy-2-carboxy-quinoline (formula VII)
1000mL hydrogenation autoclave replaced with nitrogen gas into6-nitro-veratraldehyde (63.4g, 0.3mol)And THF (380 mL),After stirring and dissolving,Add 5% Pd/C (6.34g, moisture content 63.5%),After the nitrogen is replaced, the hydrogen pressure is introduced to 0.1 to 0.2 MPa.The reaction was kept at 25-30 ° C for 4 hours.Vent to atmospheric pressure,The catalyst was filtered off by filtration.The filtrate was transferred to a 1000 mL four-neck bottle.Add ethyl pyruvate (38.3 g, 0.33 mol),Warming up to 60 ° C,Sodium ethoxide solid (30.6 g, 0.45 mol) was added in portions.After the addition, the reaction was kept at 60-65 ° C for 4 hours.Add water (200mL),The reaction was continued at 60-65 ° C for 2 hours.After cooling, the mixture was evaporated to remove most of the THF.The residue was extracted with water (300 mL) and tert-ether (200 mL).Collect the water layer,Adjust the pH to 6-7 with 10% hydrochloric acid.A large amount of solid precipitated.Filter and filter the filter cake with water (100 mL).The filter cake was dried at 70 ° C overnight to obtain 6,7-dimethoxy-2-carboxy-quinoline.(Formula VII, yellow solid, 57.8 g, 82.6%)
References:
CN110117254,2019,A Location in patent:Paragraph 0021; 0022; 0023; 0024; 0025; 0026

64-19-7
1628 suppliers
$10.00/25ML
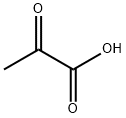
127-17-3
565 suppliers
$5.00/10g
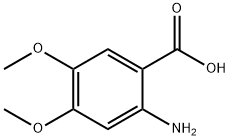
5653-40-7
285 suppliers
$12.50/5G

122234-49-5
4 suppliers
inquiry

20357-25-9
282 suppliers
$5.00/25mg

122234-49-5
4 suppliers
inquiry
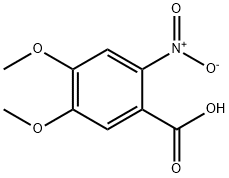
4998-07-6
232 suppliers
$6.00/5g

122234-49-5
4 suppliers
inquiry