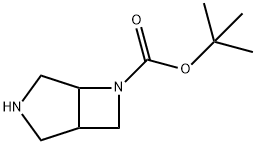
6-BOC-3,6-DIAZABICYCLO[3.2.0]HEPTANE synthesis
- Product Name:6-BOC-3,6-DIAZABICYCLO[3.2.0]HEPTANE
- CAS Number:122848-57-1
- Molecular formula:C10H18N2O2
- Molecular Weight:198.26
![(1R,5S)-3-benzyl 6-tert-butyl 3,6-diazabicyclo[3.2.0]heptane-3,6-dicarboxylate](/CAS/GIF/370880-79-8.gif)
370880-79-8
![6-BOC-3,6-DIAZABICYCLO[3.2.0]HEPTANE](/CAS/GIF/122848-57-1.gif)
122848-57-1
3-Benzyl-6-tert-butyl-3,6-diazabicyclo[3.2.0]heptane-3,6-dicarboxylate (2.61 mmol) was used as a raw material and dissolved in methanol (0.1 M) in a Parr vial. Palladium/carbon catalyst (Johnson Mathey, 5% wt, 61.1% H2O, 60 mg) was added. The mixture was placed on a Parr shaker and the air was removed by vacuum. The system was purged repeatedly with nitrogen followed by shaking at 15 PSI for about 1.5 hr. The progress of the reaction was monitored by TLC to confirm complete consumption of raw materials. Upon completion of the reaction, the mixture was filtered through a diatomaceous earth pad. The filtrate was concentrated to afford a viscous clear residue, tert-butyl 3,6-diazabicyclo[3.2.0]heptane-6-carboxylate, in 66.1% yield. The product was sufficiently pure to be used in subsequent reactions without further purification. Mass spectrometry (ESI) calculated value: C10H18N2O2, 198.26; measured value: m/z 199.2 [M + H]+. 1H NMR (400 MHz, CDCl3) δ 4.73-4.50 (m, 1H), 3.98 (t, J = 8.3 Hz, 1H), 3.46-3.15 (m, 2H), 3.09-2.99 (m , 1H), 2.93-2.82 (m, 1H), 2.73-2.62 (m, 1H), 2.53-2.42 (m, 1H), 2.31 (s, 1H), 1.43 (s, 9H).
![(1R,5S)-3-benzyl 6-tert-butyl 3,6-diazabicyclo[3.2.0]heptane-3,6-dicarboxylate](/CAS/GIF/370880-79-8.gif)
370880-79-8
19 suppliers
$504.38/5MG
![6-BOC-3,6-DIAZABICYCLO[3.2.0]HEPTANE](/CAS/GIF/122848-57-1.gif)
122848-57-1
72 suppliers
$97.00/50mg
Yield:122848-57-1 66.1%
Reaction Conditions:
with hydrogen;5% Pd(II)/C(eggshell) in methanol under 775.743 Torr; for 1.5 h;
Steps:
18.I
3-Benzyl, 6-tert-butyl-3,6-diazabicyclo[3.2.0]heptane-3,6-dicarboxylate (2.61 mmol) in a Parr bottle was taken up in MeOH (0.1 M). Pd/C (5%wt- 61 .1 % H2O from Johnson Mathey, 60 mg) was added. The mixture was placed on the Parr shaker and air was removed via vacuum. The system was purged a few time with nitrogen and then allowed to shake at 15 PSI for ~1 .5 hr. TLC showed that all the starting material was consumed. The mixture was filtered through a pad of Celite. The filtrate was concentrated to provide a viscous clear residue, II in 66.1 %. Material was clean enough to carry forward without purification. MS (ESI) mass calcd. for Ci0Hi8N2O2, 198.26; m/z found, 199.2 [M+H]+. 1H NMR (400 MHz, CDCI3) 4.73 - 4.50 (m, 1 H), 3.98 (t, J = 8.3, 1 H), 3.46 - 3.15 (m, 2H), 3.09 - 2.99 (m, 1 H), 2.93 - 2.82 (m, 1 H), 2.73 - 2.62 (m, 1 H), 2.53 - 2.42 (m, 1 H), 2.31 (s, 1 H), 1 .43 (s, 9H).
References:
JANSSEN PHARMACEUTICA NV;BRANSTETTER, Bryan, James;LETAVIC, Michael, A.;LY, Kiev, S.;RUDOLPH, Dale, A.;SAVALL, Brad, M.;SHAH, Chandravadan, R.;SHIREMAN, Brock, T. WO2011/50200, 2011, A1 Location in patent:Page/Page column 60-61

1017789-40-0
17 suppliers
$90.00/50mg
![6-BOC-3,6-DIAZABICYCLO[3.2.0]HEPTANE](/CAS/GIF/122848-57-1.gif)
122848-57-1
72 suppliers
$97.00/50mg
1255099-67-2
23 suppliers
$65.00/10mg
![6-BOC-3,6-DIAZABICYCLO[3.2.0]HEPTANE](/CAS/GIF/122848-57-1.gif)
122848-57-1
72 suppliers
$97.00/50mg
![3-CBZ-3,6-DIAZABICYCLO[3.2.0]HEPTANE](/CAS/GIF/370880-87-8.gif)
370880-87-8
49 suppliers
$296.80/250mg
![6-BOC-3,6-DIAZABICYCLO[3.2.0]HEPTANE](/CAS/GIF/122848-57-1.gif)
122848-57-1
72 suppliers
$97.00/50mg
569682-60-6
26 suppliers
inquiry
![6-BOC-3,6-DIAZABICYCLO[3.2.0]HEPTANE](/CAS/GIF/122848-57-1.gif)
122848-57-1
72 suppliers
$97.00/50mg