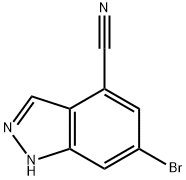
6-Bromo-1H-indazole-4-carbonitrile synthesis
- Product Name:6-Bromo-1H-indazole-4-carbonitrile
- CAS Number:898747-00-7
- Molecular formula:C8H4BrN3
- Molecular Weight:222.04
Yield:1245465-10-4 370 mg
Reaction Conditions:
Stage #1: 6-bromo-1H-indazole-4-carbonitrilewith sodium hydride in tetrahydrofuran at 0; for 0.166667 h;
Stage #2: methyl iodide in tetrahydrofuran at 0 - 0.2; for 20 h;
Steps:
6-Bromo-1-methyl-1H-indazole-4-carbonitrile
6-Bromo-1-methyl-1H-indazole-4-carbonitrile
Tetrahydrofuran (27 ml) was added to a flask containing sodium hydride (0.275 g, 6.89 mmol) and the mixture was stirred for 10 minutes at 0° C. 6-Bromo-1H-indazole-4-carbonitrile (1.39 g, 6.26 mmol) was added portionwise and the mixture was stirred for 10 mins until no further effervescence was seen.
Iodomethane (0.431 ml, 6.89 mmol) was added and the mixture stirred at 0° C. for 1 h.
The ice bath was removed and the flask was placed in a water bath at room temperature.
The reaction remained stirring for 19 h and the mixture was then evaporated in vacuo.
The residual solid purified by silica (100 g) cartridge using a gradient of ethyl acetate and cyclohexane to give the title compound as a white solid (370 mg). LCMS (Method B): Rt 2.60 mins, MH+ 237.
References:
US9326987,2016,B2 Location in patent:Page/Page column 99

898747-00-7
58 suppliers
inquiry

1245464-71-4
0 suppliers
inquiry

898747-00-7
58 suppliers
inquiry
![1H-Indazole, 4-[5-(chloromethyl)-1,3,4-oxadiazol-2-yl]-6-(1H-indol-4-yl)-1-(phenylsulfonyl)-](/CAS/20210305/GIF/1245464-74-7.gif)
1245464-74-7
0 suppliers
inquiry