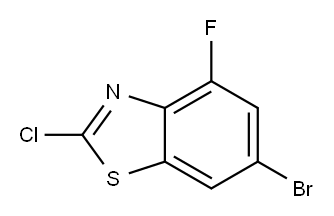
6-BROMO-2-CHLORO-4-FLUOROBENZOTHIAZOLE synthesis
- Product Name:6-BROMO-2-CHLORO-4-FLUOROBENZOTHIAZOLE
- CAS Number:960535-41-5
- Molecular formula:C7H2BrClFNS
- Molecular Weight:266.52
Yield:960535-41-5 95.3%
Reaction Conditions:
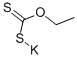
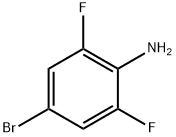
Stage #1: potassium ethyl xanthogenate;4-bromo-2,6-difluoroaniline in N,N-dimethyl-formamide at 130;
Stage #2: with sulfuryl dichloride in dichloromethane at 20; for 48 h;
Steps:
1.1 Step 1
A mixture of 4-bromo-2,6-difluoro-aniline (4.16 g, 20.0 mmol, 1.00 eq.) and ethoxycarbothioylsulfanyl potassium (7.69 g, 48.0 mmol, 2.40 eq.) in DMF (25 mL) was stirred at 130°C overnight, then cooled to room temperature, diluted with 1 N HCl (150 mL) and stirred at room temperature for 1 h. The resulting solid was filtered and washed with water and dried. The resulting material was suspended in CH2Cl2 (25 mL) and SO2Cl2 (27.5 g, 16.5 mL, 200 mmol, 10.0 eq.) was added slowly and stirred at room temperature for 48 h. Water was added slowly at 0° C to quenched the reaction. The resulting precipitate was collected by filtration and purified over silica with ethyl acetate in hexanes (2 to 10% gradient) to give 6-bromo-2-chloro-4-fluoro- 1,3-benzothiazole (5.08 g, 95.3%). MS m/z 266.1, 268.0, 270.0 [M+H]+.
References:
WO2020/5877,2020,A1 Location in patent:Page/Page column 149
367-24-8
509 suppliers
$15.00/25g

960535-41-5
12 suppliers
inquiry