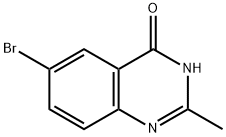
6-BROMO-2-METHYL-3,4-DIHYDROQUINAZOLIN-4-ONE synthesis
- Product Name:6-BROMO-2-METHYL-3,4-DIHYDROQUINAZOLIN-4-ONE
- CAS Number:5426-59-5
- Molecular formula:C9H7BrN2O
- Molecular Weight:239.07

108-24-7
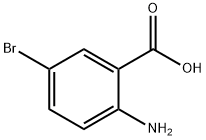
5794-88-7

5426-59-5
The general procedure for the synthesis of 6-bromo-2-methylquinazolin-4(3H)-one from ethanoic anhydride and 2-amino-5-bromobenzoic acid was as follows: first, 2-amino-5-bromobenzoic acid was refluxed for 1 h in liquid ammonia (10 mL/g). Subsequently, the reaction solution was concentrated to a smaller volume and the residue was mixed with acetic anhydride (2 mL/g) and refluxing was continued for 4 hours. Upon completion of the reaction, water was added to the mixture and boiled for 30 minutes, followed by dropwise addition of 20% sodium hydroxide solution until a clarified solution was formed. Next, ammonium carbonate was added to the hot solution until a precipitate was formed. The mixture was allowed to stand at 4°C for 16 hours, after which the precipitate was collected by filtration, washed with water and finally dried under vacuum to give the target product in about 60% yield.

64-17-5
825 suppliers
$10.00/50g
16313-66-9
107 suppliers
$5.00/100mg

5426-59-5
91 suppliers
$22.00/250mg
Yield: 97%
Reaction Conditions:
with tert.-butylhydroperoxide in dimethyl sulfoxide at 25 - 35; for 40 h;Irradiation;Green chemistry;
Steps:
1. General information. General experimental procedure for the synthesis of quinazolinones:
General procedure: a 10-mL glass tube was filled with the required 2-aminobenzamide (0.2 mmol), alcohol (0.5 mmol), and TBHP (1 equiv.) and irradiated at room temperature with an 18 W blue LED for 40 h. After reaction completion (monitored by TLC), water was added to the reaction solution and the mixture was extracted with EtOAc. The combined organic layers were dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude product was purified by silica-gel column chromatography using petroleum ether/ethyl acetate as eluent to give the desired product.
References:
Xie, Zongbo;Lan, Jin;Zhu, Haibo;Lei, Gaoyi;Jiang, Guofang;Le, Zhanggao [Chinese Chemical Letters,2021,vol. 32,# 4,p. 1427 - 1431] Location in patent:supporting information

108-24-7
0 suppliers
$14.00/250ML

5794-88-7
474 suppliers
$6.00/10g

5426-59-5
91 suppliers
$22.00/250mg
19165-25-4
50 suppliers
$59.10/250mg

5426-59-5
91 suppliers
$22.00/250mg

189634-99-9
7 suppliers
inquiry

5426-59-5
91 suppliers
$22.00/250mg
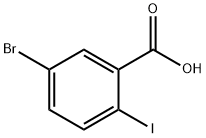
21740-00-1
335 suppliers
$8.00/5g
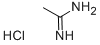
124-42-5
372 suppliers
$6.00/25g

5426-59-5
91 suppliers
$22.00/250mg