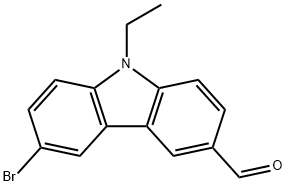
6-bromo-9-ethyl-9H-carbazole-3-carbaldehyde synthesis
- Product Name:6-bromo-9-ethyl-9H-carbazole-3-carbaldehyde
- CAS Number:24301-72-2
- Molecular formula:C15H12BrNO
- Molecular Weight:302.17
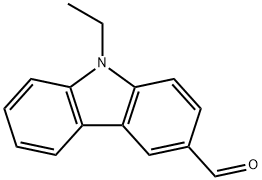
7570-45-8
200 suppliers
$11.00/1g

24301-72-2
7 suppliers
inquiry
Yield:24301-72-2 87%
Reaction Conditions:
with N-Bromosuccinimide in chloroform;acetic acid at 20; for 15 h;Inert atmosphere;
Steps:
6-bromo-9-ethyl-9H-carbazole-3-carbaldehyde (3):
9.8 mmol (2.2 g) N-ethyl-3-aldehyde carbazole and 10.67 mmol (1.09 g) N-bromosuccinimide (NBS) were added into 30 mL trichloromethane and 30 mL glacial acetic acid. The reaction was stirred at room temperature for 15 hours under nitrogen protection then pour into 100mL deionized water and extracted with dichloromethane. The organic phase was separated, dried over anhydrous sodium sulfate and evaporated under reduced pressure at 50 . The remaining was purified with silica (dichloromethane/petroleum ether 1:1) to get he white solid product 25.28 mg with a yield of 87%.1H NMR (400 MHz, CDCl3) δ 10.08 (s, 1H), 8.55 (d, J = 32.2 Hz, 1H), 8.23 (d, J = 1.7 Hz, 1H), 8.02 (dd, J = 8.5, 1.3 Hz, 1H), 7.60 (dd, J = 8.6, 1.8 Hz, 1H), 7.46 (d, J = 8.5 Hz, 1H), 7.32 (d, J = 8.6 Hz, 1H), 4.36 (dd, J = 14.5, 7.2 Hz, 2H), 1.45 (t, J = 7.2 Hz, 3H).
References:
Chen, Yan;Mo, Zhao;Zhu, Xingtong;Xu, Qingxiang;Xue, Zhaoli;Li, Henan;Xu, Hui;Zhao, Long [Journal of Alloys and Compounds,2022,vol. 889,art. no. 161795] Location in patent:supporting information
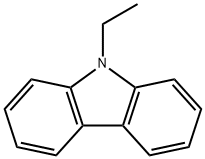
86-28-2
393 suppliers
$9.00/25g

24301-72-2
7 suppliers
inquiry
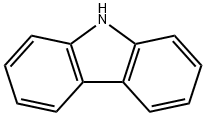
86-74-8
347 suppliers
$10.00/5g

24301-72-2
7 suppliers
inquiry