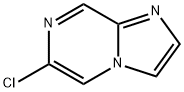
6-Chloro-imidazo[1,2-a]pyrazine synthesis
- Product Name:6-Chloro-imidazo[1,2-a]pyrazine
- CAS Number:76537-23-0
- Molecular formula:C6H4ClN3
- Molecular Weight:153.57
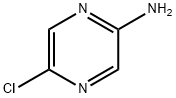
33332-29-5
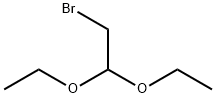
2032-35-1
![6-Chloro-imidazo[1,2-a]pyrazine](/CAS/GIF/76537-23-0.gif)
76537-23-0
2-Amino-5-chloropyrazine (2.6 g, 20 mmol) and 2-bromo-1,1-diethoxyethane (12.0 g, 60.89 mmol) were used as starting materials and dissolved in 30 mL of isopropanol. Subsequently, hydrogen bromide solution (10.5 g, 62.22 mmol) was added to the reaction system and the reaction was stirred at 80°C overnight. Upon completion of the reaction, the mixture was cooled to room temperature and the pH was adjusted with sodium bicarbonate to 8. Next, extraction was carried out with dichloromethane, the organic phases were separated and combined, and the crude product was concentrated under reduced pressure. Purification by Combi-flash fast column chromatography (eluent ratio: dichloromethane:methanol = 90:10:70:30) afforded a brown solid product, 6-chloroimidazo[1,2A]pyrazine (2.3 g), in a yield of 49.75% and a purity of 96.63%. The product did not need further purification and could be used directly in the subsequent reaction.

33332-29-5
262 suppliers
$14.00/1g

2032-35-1
488 suppliers
$5.00/10g
![6-Chloro-imidazo[1,2-a]pyrazine](/CAS/GIF/76537-23-0.gif)
76537-23-0
116 suppliers
$40.00/1g
Yield: 49.75%
Reaction Conditions:
with hydrogen bromide in isopropyl alcohol at 80;
Steps:
16.1 Step 1:
compound 5-a (2.6g, 20mmol), 2- bromo-1,1-diethoxyethane (12.0g, 60.89mmol) was dissolved in 30ml isopropanol was then added a solution of hydrogen bromide (10.5g, 62.22mmol), 80 deg. C stirred overnight. Completion of the reaction, cooled to room temperature, adjusted to pH 8 sodium hydrogen carbonate, extracted with dichloromethane, the combined organic phases separated, concentrated under reduced pressure to give a crude product which, by Combi-flash chromatography [DCM: MeOH = 90: 10 ~ 70: 30 ] to give a brown solid compound 16-b (2.3g), was used directly in the next reaction. Yield: 49.75%, purity 96.63%.
References:
Shanghai Haiyan Pharmaceutical Technology Co. Ltd;Yangtze River Pharmaceutical Group Co., Ltd;Jin, Yunzhou;Bo, Ping;He, Qi;Lan, Jiong;Zhou, Fusheng;Zhang, Liang;He, Xiangyu CN105524068, 2016, A Location in patent:Paragraph 0286; 0287
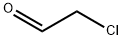
107-20-0
0 suppliers
$18.10/5ml

33332-29-5
262 suppliers
$14.00/1g
![6-Chloro-imidazo[1,2-a]pyrazine](/CAS/GIF/76537-23-0.gif)
76537-23-0
116 suppliers
$40.00/1g