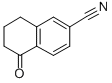
6-CYANO-1-TETRALONE synthesis
- Product Name:6-CYANO-1-TETRALONE
- CAS Number:90401-84-6
- Molecular formula:C11H9NO
- Molecular Weight:171.2
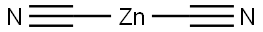
557-21-1
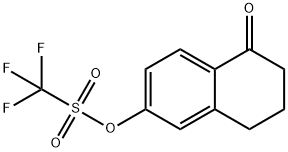
144464-64-2

90401-84-6
Step 2: Synthesis of 5-oxo-5,6,7,8-tetrahydronaphthalene-2-carbonitrile A solution of 5-oxo-5,6,7,8-tetrahydronaphthalen-2-yl trifluoromethanesulfonate (11.5 g, 39.0 mmol) prepared in the previous step was degassed with zinc cyanide (2.7 g, 23.5 mmol) in anhydrous dimethylformamide (100 mL) and reacted under nitrogen atmosphere. To the reaction mixture was added tetrakis(triphenylphosphine)palladium(0) (1.7 g, 1.5 mmol), which was again degassed and kept under nitrogen atmosphere. The reaction mixture was stirred at 135 °C overnight. Subsequently, tetrakis(triphenylphosphine)palladium (0) (171 mg) was added supplementally and stirring was continued for 4 hours. Upon completion of the reaction, the reaction mixture was filtered through Celite? and rinsed with ethyl acetate. The filtrate was washed with water, the organic layer was separated and dried over anhydrous sodium sulfate. The dried organic layer was filtered and concentrated in vacuum to give 8.1 g of crude product. The crude product was purified by silica gel column chromatography using a gradient elution of 5% to 15% ethyl acetate/hexane, resulting in 5-oxo-5,6,7,8-tetrahydronaphthalene-2-carbonitrile (2.8 g, 41% yield) with a mass spectrometry (ES) m/z 172 [M + H]+.
Yield: 41%
Reaction Conditions:
tetrakis(triphenylphosphine) palladium(0) in N,N-dimethyl-formamide at 135;
Steps:
6.2
Step 2: 5-Oxo-5,6,7,8-tetrahydro-naphthalene-2-carbonitrile A mixture of 5-oxo-5,6,7,8-tetrahydronaphthalen-2-yl trifluoromethanesulfonate (11.5 g, 39.0 mmol), prepared in the previous step, and zinc cyanide (2.7 g, 23.5 mmol) in dry dimethylformamide (100 mL) was degassed and put under a nitrogen atmosphere. Tetrakis(triphenylphosphine)palladium(0) (1.7 g, 1.5 mmol) was added and the mixture again degassed and put under a nitrogen atmosphere. The mixture was stirred at 135° C. overnight. An additional 171 mg of tetrakis(triphenylphosphine)palladium(0) was added and the reaction stirred for another 4 h. The reaction mixture was filtered through the Celite reagent and rinsed with ethyl acetate. The filtrate was washed with water. The layers were separated and the organic layer was dried over anhydrous sodium sulfate. The organic layer was filtered and concentrated in vacuo to give 8.1 g of crude product. Purification of the crude product on silica gel using a step-wise gradient of 5% to 15% ethyl acetate: hexane as the eluent gave 5-oxo-5,6,7,8-tetrahydro-naphthalene-2-carbonitrile (2.8 g, 41%), MS(ES) m/z 172 [M+H]+.
References:
Wyeth US2008/45578, 2008, A1 Location in patent:Page/Page column 18
3470-53-9
219 suppliers
$6.00/250mg
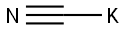
151-50-8
0 suppliers
$36.39/100g

90401-84-6
77 suppliers
inquiry
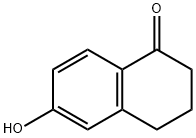
3470-50-6
233 suppliers
$6.00/1g

90401-84-6
77 suppliers
inquiry
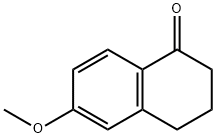
1078-19-9
380 suppliers
$6.00/5g

90401-84-6
77 suppliers
inquiry