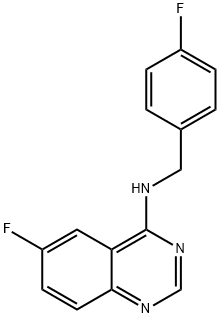
6-fluoro-N-(4-fluorobenzyl)quinazolin-4-aMine synthesis
- Product Name:6-fluoro-N-(4-fluorobenzyl)quinazolin-4-aMine
- CAS Number:1262888-28-7
- Molecular formula:C15H11F2N3
- Molecular Weight:271.26
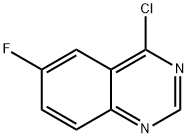
16499-61-9
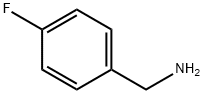
140-75-0

1262888-28-7
GENERAL METHOD: 4-Chloro-6-fluoroquinazoline (1.84 g, 10 mmol), trimethylamine (1.06 g, 10 mmol) were mixed with p-fluorobenzylamine (10 mmol) in 5 mL N,N-dimethylformamide (DMF). The reaction mixture was heated to reflux for 4 h, subsequently cooled to room temperature and decanted into crushed ice. The precipitated solid was collected by filtration and purified by recrystallization with ethanol. The melting points of the resulting 6-fluoro-N-(4-fluorobenzyl)quinazolin-4-amines were all above 300 °C.

16499-61-9
121 suppliers
$27.00/100mg

140-75-0
476 suppliers
$10.00/10g

1262888-28-7
111 suppliers
$25.00/1mg
Yield: 62%
Reaction Conditions:
with triethylamine in N,N-dimethyl-formamide for 4 h;Reflux;
Steps:
3.5. General Method of the Synthesis of Quinazoline Derivatives (5a-j)
General procedure: Compound 4 (1.84 g, 0.01 mol) was mixed with (1.06 g, 0.01 mol) of trimethylamine and (0.01 mol)of the appropriate aromatic amine in 5 mL of dimethylformamide (DMF). The mixture was refluxedfor 4 h, cooled, and poured on crushed ice. The crystals were collected and crystallized from ethylalcohol. The melting points for all synthesized compounds were above 300 °C.
References:
Zayed, Mohamed F.;Ihmaid, Saleh K.;Ahmed, Hany E.A.;El-Adl, Khaled;Asiri, Ahmed M.;Omar, Abdelsattar M. [Molecules,2017,vol. 22,# 2,art. no. 188]
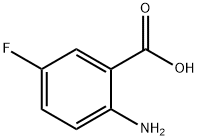
446-08-2
404 suppliers
$14.00/5g

1262888-28-7
111 suppliers
$25.00/1mg
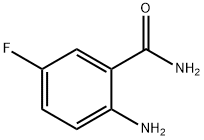
63069-49-8
144 suppliers
$15.19/250mg

1262888-28-7
111 suppliers
$25.00/1mg
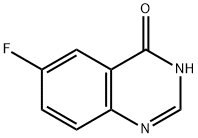
16499-56-2
109 suppliers
$12.00/250mg

1262888-28-7
111 suppliers
$25.00/1mg