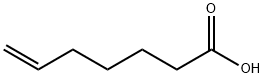
6-Heptenoic acid synthesis
- Product Name:6-Heptenoic acid
- CAS Number:1119-60-4
- Molecular formula:C7H12O2
- Molecular Weight:128.17
Synthesis of 6-Heptenoic Acid
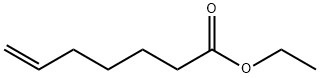
25118-23-4
61 suppliers
$27.00/250mg

1119-60-4
83 suppliers
$39.00/250mg
Yield:1119-60-4 91.8%
Reaction Conditions:
Stage #1:ethyl 6-heptenoate with sodium hydroxide in ethanol;water for 4 h;Reflux;
Stage #2: with hydrogenchloride in water; pH=5
Steps:
1 Hept-6-enoic acid (52a):
Hept-6-enoic acid (52a):To a stirred solution of Ethyl hept-6-enoate (52) (3 g, 19.2 mmol) in EtOH (100 mL), IN NaOH (7.6 g, 192.3 mmol) was added and refluxed for 4 h. The volatiles from the reaction were removed under reduced pressure, the residue was acidified (pH 5) with IN HC1 and extracted with CH2CI2 (3 x 25 mL). The combined organic extracts were dried over anhydrous Na2S04, filtered and concentrated under reduced pressure to afford compound 52a (2.26 g, 91.8%) as colorless liquid.TLC: 20% EtOAc/Hexane (Rf: 0.1)1H NMR (500MHz, CDC13): δ 5.83-5.75 (m, 1H), 5.06 (dd, 7 = 17, 1.5 Hz, 2H), 2.39 (t, J = 7.5 Hz, 2H), 2.09-2.04 (m, 2H), 1.66-1.60 (m, 2H), 1.47-1.43 (m, 2H).Mass (ESI): 127 (M+-l).
References:
THERACRINE, INC.;SUN, Lijun;BARSOUM, James;WESTER, Ronald WO2013/13240, 2013, A2 Location in patent:Page/Page column 58

64-19-7
1628 suppliers
$10.00/25ML
1119-51-3
391 suppliers
$12.00/5g

1119-60-4
83 suppliers
$39.00/250mg

124-38-9
134 suppliers
$175.00/23402

2695-47-8
293 suppliers
$14.00/5g

1119-60-4
83 suppliers
$39.00/250mg
![Bicyclo[4.1.0]heptane, 1-[(trimethylsilyl)oxy]-](/CAS/20210305/GIF/38858-74-1.gif)
38858-74-1
0 suppliers
inquiry

1119-60-4
83 suppliers
$39.00/250mg
505-48-6
447 suppliers
$8.00/10g

1119-60-4
83 suppliers
$39.00/250mg