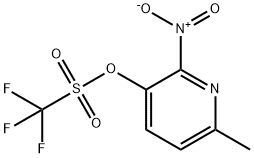
6-METHYL-2-NITRO-3-PYRIDYL TRIFLUOROMET& synthesis
- Product Name:6-METHYL-2-NITRO-3-PYRIDYL TRIFLUOROMET&
- CAS Number:163083-48-5
- Molecular formula:C7H5F3N2O5S
- Molecular Weight:286.19
Yield:163083-48-5 98%
Reaction Conditions:
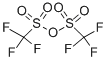
with triethylamine in dichloromethane at 0; for 2 h;
Steps:
1 6-METHYL-2-NITROPYRIDIN-3-YL TRIFLUOROMETHANESULFONATE
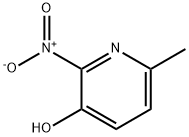
Step1 6-METHYL-2-NITROPYRIDIN-3-YL TRIFLUOROMETHANESULFONATE To a solution of 3-hydroxy-6-methyl-2-nitropyridine (2 g, 12.97 MMOL, 1 eq) in CH2CI2 (150 mL) at 0 C under N2 was added triethylamine (2.68 mL, 19.27 mmol, 1.48 eq) and followed BY TRIFLUOROMETHANESULFONIC anhydride (2.62 mL, 15.57 mmol, 1.2 eq). The mixture was stirred for 2 hours at 0 C and then quenched with water. The organic layer was separated, washed with water and dried over MGS04. After filtration and concentration at reduced pressure, the crude mixture was purified by flash chromatography on silica gel (15% EA/Hex) to afford the desired product (3.65 g, 98% yield) as a yellow oil. H NMR (CDCI3) 8 2.70 (s, 3H), 7.59 (d, 1H), 7.81 (d, 2H).
References:
WO2004/58760,2004,A1 Location in patent:Page 59