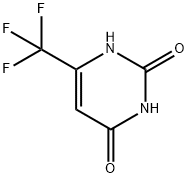
6-(TRIFLUOROMETHYL)URACIL synthesis
- Product Name:6-(TRIFLUOROMETHYL)URACIL
- CAS Number:672-45-7
- Molecular formula:C5H3F3N2O2
- Molecular Weight:180.08
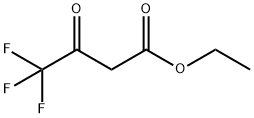
372-31-6
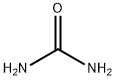
57-13-6

672-45-7
Ethyl 4,4,4-trifluoro-3-oxobutanoate (3 g, 16.30 mmol) was dissolved in ethanol (30 mL) under argon protection and urea (978 mg, 16.30 mmol) was added with stirring. Subsequently, freshly prepared sodium ethoxide solution (made by reacting 750 mg of sodium metal with 30 mL of anhydrous ethanol) was added to the reaction system. The reaction mixture was heated to 90 °C and the reaction was stirred at this temperature for 24 hours. The reaction process was monitored by thin layer chromatography (TLC). Upon completion of the reaction, volatile solvents were removed by distillation under reduced pressure, and the residue was diluted with water (25 mL), acidified with 1N hydrochloric acid (10 mL) to pH < 7, and then extracted with ethyl acetate (2 x 40 mL). The organic phases were combined, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give the target product 6-(trifluoromethyl)uracil (650 mg, 22% yield) as a white solid. Thin layer chromatographic conditions: 5% methanol/dichloromethane (Rf=0.3).1H-NMR (DMSO-d6, 500MHz) data: δ 11.07 (s, 1H), 11.54 (s, 1H), 6.07 (s, 1H).
Yield: 22%
Reaction Conditions:
with sodium ethanolate in ethanol at 20 - 90; for 24 h;Inert atmosphere;
Steps:
11 Synthesis of
To a stirred solution of ethyl 4.4,44rifluoro-3-oxobutanoate 101 (3 g. 1 &30 nim-ol) in ethin.iol (30 mL) under argon atmosphere were added urea 102- (978 mg, 16.30 mmol) and freshly prepared sodium ethoxide (750 mg of Na in 30 mL of EtOH) at Pt; the mixture was heated to 90 °C and stirred for 24 h. The reaction was monitored by TIE; after completion of the reaction, the volati Ic-s were removed in vacua, diluted with water (25 mL), acidified with 1 N SC1 UO niL) and extracted with EtOAc (2 x 40 niL). The comb med organic extracts were dried over sodium sulfate, filtered and concentrated in vacua to afford compound 103 (650 mg, 22%) as a white solid. TL-C: 5% CH3OR CK2CI2 (1?,: 0.3); ‘H-NMR (DMSO-%, 500 MHz): 6 1107 (s. 114), i1.54(s. iS). 6.07 (s, 1H
References:
INDIANA UNIVERSITY RESEARCH AND TECHNOLOGY CORPORATION;ZLOTNICK, Adam;LI, Lichun;TURNER, William Jr.;FRANCIS, Samson WO2015/57945, 2015, A1 Location in patent:Paragraph 00121
![[[1,4-DIHYDRO-4-OXO-6-(TRIFLUOROMETHYL)-2-PYRIMIDINYL]THIO]-ACETIC ACID](/CAS/GIF/836-12-4.gif)
836-12-4
12 suppliers
inquiry

672-45-7
151 suppliers
$14.00/1g
175354-56-0
50 suppliers
$45.00/250mg

672-45-7
151 suppliers
$14.00/1g
368-54-7
162 suppliers
$5.00/250mg

672-45-7
151 suppliers
$14.00/1g
16097-62-4
111 suppliers
$13.00/1g

672-45-7
151 suppliers
$14.00/1g