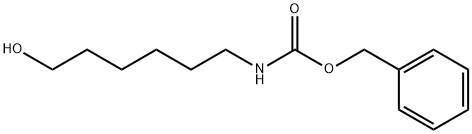
6-(Z-AMINO)-1-HEXANOL synthesis
- Product Name:6-(Z-AMINO)-1-HEXANOL
- CAS Number:17996-12-2
- Molecular formula:C14H21NO3
- Molecular Weight:251.32

4048-33-3

501-53-1

17996-12-2
General procedure: 6-amino-1-hexanol (5.0 g, 42.7 mmol) was dissolved in a solvent mixture of tetrahydrofuran (50 mL) and water (15 mL), and benzyl chloroformate (7.3 mL, 51.2 mmol) was added slowly and dropwise at 0°C. The pH of the reaction solution was adjusted to 8-9 by adding 1N sodium hydroxide solution (12 mL). Under the condition of maintaining the temperature of the reaction system at 0 °C, 1N sodium hydroxide solution (12 mL) was slowly added to adjust the pH of the reaction solution to 8-9, a process that took about 30 min. Subsequently, the reaction mixture was continued to be stirred at room temperature for 30 minutes. Upon completion of the reaction, the reaction was extracted with ether (30 mL x 3), the organic phases were combined, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The resulting crude product was recrystallized by solvent recrystallization of a mixture of dichloromethane and hexane to afford N-Cbz-6-amino-1-hexanol as a white crystalline solid (3.85 g, 90% yield). The structure of the product was confirmed by 1H NMR and 13C NMR: 1H NMR (400 MHz, CDCl3): δ7.34 (m, 5H, Ar-H), 5.07 (s, 2H, PhCH2), 4.73 (br, 1H, NH), 3.61 (t, J=6.4Hz, 2H, CH2O), 3.16 (dt, J=6.8,6.8 Hz, 2H, NCH2), 1.61-1.32 (m, 8H, CH2); 13C NMR (100 MHz, CDCl3): δ 156.4 (C=O), 136.6,128.5,128.1 (Ar-C), 66.6 (PhCH2), 62.7 (CH2OH), 40.9 (NHCH2), 32.5 ( CH2CH2OH), 29.9 (NHCH2CH2), 26.3 (CH2CH2CH2OH), 25.3 (NCH2CH2CH2).
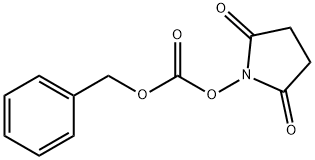
13139-17-8
516 suppliers
$6.00/25g

4048-33-3
347 suppliers
$10.00/1g

17996-12-2
96 suppliers
$18.00/250mg
Yield:17996-12-2 83%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 1 h;Inert atmosphere;
Steps:
26
00568] Compound 1001 - Starting compound 1000 (10 g, 85.38 mmol) was added to the reaction flask, evacuated and purged with argon. The starting material was dissolved in dichloromethane and triethylamine (23.79 rriL, 170.7 mmol) was added via syringe. N- (Benzyloxycarbonyloxy)succinimide (31.9 g, 128 mmol) was dissolved in anhydrous dichloromethane and then added to the reaction mixture via syringe. The reaction was stirred at room temperature for 1 hour. The reaction was checked by TLC (50% EtOAc/hexanes) and the reaction was concentrated under reduced pressure. The residue was dissolved in dichloromethane, added to separation funnel and organic layer was washed with 10% citric acid solution. The organic layer was separated and washed with a brine solution. The organic layer was separated and dried with sodium sulfate. The solid was filtered off and the mother liquor was concentrated. The residue was purified by flash chromatography on silica gel (10% to 100% EtOAc/hexanes) and the product fractions combined and concentrated on reduced pressure to yield 17.9g, (83%) of 1001. NMR (400 MHz, DMSO-de): δ 7.40 - 7.25 (m, 4H), 7.21 (t, J = 5.7 Hz, 1H), 4.99 (s, 2H), 4.31 (t, J = 5.1 Hz, 1H), 3.36 (m, 2H), 2.96 (q, J = 6.7 Hz, 2H), 1.38 (m, 4H), 1.32 - 1.17 (m, J = 5.2, 4.6 Hz, 4H).
References:
WO2018/136620,2018,A2 Location in patent:Paragraph 00568

4048-33-3
347 suppliers
$10.00/1g

501-53-1
408 suppliers
$10.00/1g

17996-12-2
96 suppliers
$18.00/250mg
![[1S,2S,(-)]-1,2-Cyclohexanedimethanol](/CAS/GIF/3205-34-3.gif)
3205-34-3
94 suppliers
$19.00/250mg

17996-12-2
96 suppliers
$18.00/250mg
1189-71-5
365 suppliers
$18.00/5g
629-11-8
484 suppliers
$6.00/25g

100-51-6
1433 suppliers
$5.00/100g

17996-12-2
96 suppliers
$18.00/250mg

4048-33-3
347 suppliers
$10.00/1g
1885-14-9
344 suppliers
$10.00/1g

17996-12-2
96 suppliers
$18.00/250mg