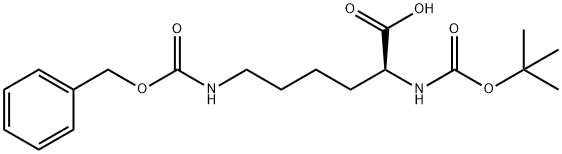
N-Boc-N'-Cbz-L-lysine synthesis
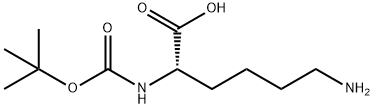
- Product Name:N-Boc-N'-Cbz-L-lysine
- CAS Number:2389-45-9
- Molecular formula:C19H28N2O6
- Molecular Weight:380.44
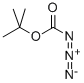
13139-17-8
13734-28-6

2389-45-9
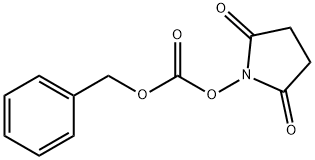
The general procedure for the synthesis of N-Boc-N'-Cbz-L-lysine from N-alpha-Boc-L-lysine and phenylmethoxycarbonylsuccinimide was as follows: to a mixed solution of (2S)-6-amino-2-(tert-butoxycarbonylamino)hexanoic acid (100.00 g, 406.01 mmol, 1.00 equiv) and NaHCO3 (102.33 g 1.22 mol, 3.00 eq.) in a mixed solution of THF (1000 mL) and water (1000 mL) was slowly added CbzOSu (101.19 g, 406.01 mmol, 1.00 eq.) at 0 °C. Subsequently, the reaction mixture was stirred at 30 °C for 16 hours. Upon completion of the reaction, the pH of the reaction mixture was adjusted to 5-6 with 1N HCl and then extracted with ethyl acetate (600 mL x 3). The organic phases were combined, dried with anhydrous Na2SO4 and concentrated under reduced pressure to afford the target product (2S)-6-(benzyloxycarbonylamino)-2-(tert-butoxycarbonylamino)hexanoic acid (142.00 g, 373.26 mmol, 91.9% yield) as a yellow oil.

13139-17-8
516 suppliers
$6.00/25g

13734-28-6
362 suppliers
$8.00/1g

2389-45-9
318 suppliers
$6.00/1g
Yield:2389-45-9 91.9%
Reaction Conditions:
with sodium hydrogencarbonate in tetrahydrofuran;water at 0 - 30; for 16 h;
Steps:
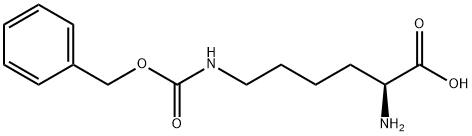
7 (2S)-6-(benzyloxycarbonylamino)-2-(tert-butoxycarbonylamino)hexanoic acid (ii)
(2S)-6-(benzyloxycarbonylamino)-2-(tert-butoxycarbonylamino)hexanoic acid (ii)
To a solution of (2S)-6-amino-2-(tert-butoxycarbonylamino)hexanoic acid (100.00 g, 406.01 mmol, 1.00 eq), NaHCO3 (102.33 g, 1.22 mol, 3.00 eq) in THF (1000 mL) and H2O (1000 mL) was added CbzOSu (101.19 g, 406.01 mmol, 1.00 eq) at 0° C.
Then the mixture was stirred at 30° C. for 16 hr.
The mixture was adjusted to pH=5-6 with 1N HCl, extracted with EA (600 mL*3), dried over Na2SO4, concentrated to give (2S)-6-(benzyloxycarbonylamino)-2-(tert-butoxycarbonylamino)hexanoic acid (142.00 g, 373.26 mmol, 91.9% yield) as a yellow oil.
References:
Cortexyme, Inc.;KONRADI, Andrei;DOMINY, Stephen S.;CRAWFORD LYNCH, Casey;COBURN, Craig;VACCA, Joseph US2016/96830, 2016, A1 Location in patent:Paragraph 0368-0369

24424-99-5
868 suppliers
$13.50/25G
1155-64-2
318 suppliers
$6.00/5g

2389-45-9
318 suppliers
$6.00/1g
![N2-[(1,1-Dimethylethoxy)carbonyl]-N6-[(phenylmethoxy)carbonyl]-L-lysine Methyl Ester](/CAS/20200401/GIF/73548-77-3.gif)
73548-77-3
36 suppliers
$26.00/1g

2389-45-9
318 suppliers
$6.00/1g