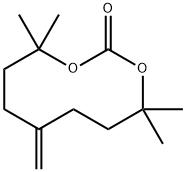
carbonic acid 2-tert-butoxycarbonyloxyMethyl-allyl ester tert-butyl ester synthesis
- Product Name:carbonic acid 2-tert-butoxycarbonyloxyMethyl-allyl ester tert-butyl ester
- CAS Number:620161-75-3
- Molecular formula:C14H24O6
- Molecular Weight:288.34
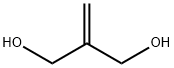
3513-81-3
214 suppliers
$10.00/1g

24424-99-5
863 suppliers
$13.50/25G

620161-75-3
4 suppliers
inquiry
Yield:620161-75-3 100%
Reaction Conditions:
with sodium hydroxide;tetra(n-butyl)ammonium hydrogensulfate in dichloromethane;water at 15 - 30; for 7 - 8 h;
Steps:
1
To a solution of di-tert-butyl dicarbonate (6.6 kg) in methylene chloride (15.0 L, 15 volumes) was added 2-methylene-1,3-propanediol (1.00 kg) and phase transfer catalyst tetrabutylammonium hydrogensulfate (0.641 kg). The resulting reaction mixture was then agitated vigorously at about 15° C. while adding over a 2 hr period, 6M aqueous sodium hydroxide solution (13.2 L) and controlling the temperature between 25 to 30° C. The resulting two-phase reaction mixture was subsequently agitated for a period of 2-3 hrs at 25° C.The aqueous layer was discarded and additional phase transfer catalyst tetrabutylammonium hydrogensulfate (0.064 kg, 10% of the initial amount), di-tert-butyl dicarbonate (0.66 kg, 10% of the initial amount), and methylene chloride (2.0 L, 2 volumes) was added to the remaining organic reaction mixture. To the reaction mixture was also added 6M aqueous sodium hydroxide solution (1.32 L, 10% of the initial amount) over a period of about 0.5 to 1 hr, while controlling the temperature between 25 to 30° C. The resulting two-phase reaction mixture was then agitated at about 25° C. for additional 3 to 4 hr. Allowing more than 3 hrs of agitation time is often required to complete the hydrolysis of the excess di-tert-butyl dicarbonate. The aqueous layer was discarded. The resulting organic phase was washed with water (3×8.0 L), diluted with EtOAc (6 L, 6 volumes), and distilled to an oil foam with quantitative yield.1H (500 MHz, CDCl3) δ 5.15, 4.98, 4.79, 4.68, 4.68, 4.33, 4.31, 3.89, 3.77, 3.67, 3.45, 3.35, 3.19, 2.88, 2.78, 2.74, 2.42, 2.17, 2.11, 2.06, 1.95, 1.72, 1.66, 1.51, 1.48, 1.43, 1.34, 1.27, 1.19, 1.19, 1.18, 1.14, 1.13, 1.11, 0.95, 0.85.
References:
US2007/207972,2007,A1 Location in patent:Page/Page column 17

3513-81-3
214 suppliers
$10.00/1g

620161-75-3
4 suppliers
inquiry

3513-81-3
214 suppliers
$10.00/1g

24424-99-5
863 suppliers
$13.50/25G
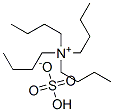
32503-27-8
666 suppliers
$5.00/25g

620161-75-3
4 suppliers
inquiry