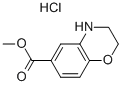
3,4-DIHYDRO-2H-BENZO[1,4]OXAZINE-6-CARBOXYLIC ACID METHYL ESTER HYDROCHLORIDE synthesis
- Product Name:3,4-DIHYDRO-2H-BENZO[1,4]OXAZINE-6-CARBOXYLIC ACID METHYL ESTER HYDROCHLORIDE
- CAS Number:648449-54-1
- Molecular formula:C10H12ClNO3
- Molecular Weight:229.6602

106-93-4
371 suppliers
$15.00/5g
536-25-4
184 suppliers
$11.00/250mg
![3,4-DIHYDRO-2H-BENZO[1,4]OXAZINE-6-CARBOXYLIC ACID METHYL ESTER HYDROCHLORIDE](/CAS/GIF/648449-54-1.gif)
648449-54-1
11 suppliers
inquiry
Yield:648449-54-1 40%
Reaction Conditions:
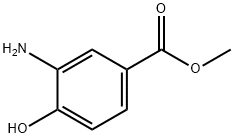
Stage #1: ethylene dibromide;methyl 3-amino-4-hydroxybenzoatewith potassium carbonate in DMF (N,N-dimethyl-formamide) at 70; for 12 h;
Stage #2: with hydrogenchloride at 20;
Steps:
61.II Step II : 3, 4-Dihydro-2H-benzo [[1,] [4] OXAZINE-6-CARBOXYLIC] acid methyl ester*hydrochloride
To a [2000ML] three-necked flask under N2 containing [3-AMINO-4-HYDROXY-BENZOIC] acid methyl ester (31. [35G,] [187MMOL)] in anhydrous DMF [(630ML ;] 20vols) at RT, was added [ K2CO3 (103G, 4EQ. ) IN ONE PORTION FOLLOWED BY 1, 2DIBROMOETHANE (65ML, 4EQ. ) IN ONE] portion. The reaction mixture was stirred at [70°C] for 12h. Temperature was allowed to cool down to RT, and [HC11N] was added until pH=8, and extraction was performed using diethyl ether (3*200ml). The organic phase was washed with water (2*200ml) and dried over [MGS04] and concentrated to afford a brown red oil with solid, which was taken up again in diethyl ether [(450ML)] and THF [(50ML)] and filtered to remove a white solid. To the filtrate was added [HC11N,] and diethyl ether [(130ML)] was added, suspension was stirred at RT for 5 minutes and filtered to give 27.6g of crude product. The aqueous phases were again extracted with ethyl acetate to afford additional 6.23g of product. The combined fractions (32g) were recrystallised from [ETOH] [(420ML] ; [13VOLS)] to give after filtration and drying a [WHITE POWDER (19. 47G (16. 37G FREE BASE) ), YIELD = 40%.] HPLC: 1.954 min. LC-MS: M/Z ESI: 1.27 min, 194.45 [(M+1).]
References:
WO2004/7491,2004,A1 Location in patent:Page 73