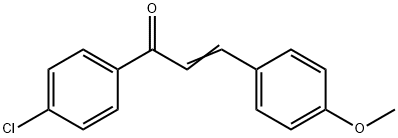
1-(4-CHLOROPHENYL)-3-(4-METHOXYPHENYL)PROP-2-EN-1-ONE synthesis
- Product Name:1-(4-CHLOROPHENYL)-3-(4-METHOXYPHENYL)PROP-2-EN-1-ONE
- CAS Number:6552-63-2
- Molecular formula:C16H13ClO2
- Molecular Weight:272.73
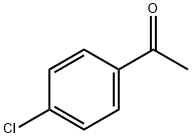
99-91-2
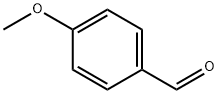
123-11-5

6552-63-2
The general procedure for the synthesis of 1-(4-chlorophenyl)-3-(4-methoxyphenyl)-2-propen-1-one from p-chloroacetophenone and p-methoxybenzaldehyde was as follows: a fresh sodium methanol/methanol solution was prepared by slicing sodium metal (0.01 g, 0.4 mmol) into thin slices and adding it slowly to anhydrous methanol (15 mL) in an ice bath until no more gas was produced. Subsequently, 4-methoxybenzaldehyde (1.36 g, 10.0 mmol) was added to the freshly prepared and cooled sodium methanol/methanol solution (15 mL), followed by the slow dropwise addition of 10.0 mmol of p-chloroacetophenone. The reaction mixture was stirred at room temperature for 2-8 hours (the reaction time was extended to 36-54 hours for substrates containing naphthalene groups or other large site-blocking groups). The reaction progress was monitored by thin layer chromatography (TLC). Upon completion of the reaction, water (20 mL) and ethyl acetate (75 mL in three additions of 25 mL each) were added to the mixture. The organic phase was separated and the aqueous phase was extracted three times with ethyl acetate. The organic phases were combined, washed three times with saturated brine and dried over anhydrous sodium sulfate. The crude product was purified by silica gel column chromatography using mixed solvent of ethyl acetate/hexane as eluent to give the pure α,β-unsaturated carbonyl compound (1-(4-chlorophenyl)-3-(4-methoxyphenyl)-2-propen-1-one).

99-91-2
429 suppliers
$10.00/25g

123-11-5
881 suppliers
$10.00/10g

6552-63-2
46 suppliers
$21.00/250mg
Yield:6552-63-2 96.9%
Reaction Conditions:
with methanol;sodium in methanol at 20;Aldol Condensation;
Steps:
General Procedure for the Synthesis of α,β-unsaturated Carbonyl Compounds (2a-2o).
General procedure: Na (0.01 g, 0.4 mmol) was cut to pieces and added into anhydrous MeOH (15 mL) under ice bath; the fresh MeONa/MeOH solution was prepared till no gas evolved. 4-Methoxybenzaldehyde (1.36 g, 10.0 mmol) was added to the fresh, cooled MeONa/MeOH solution (15 mL), and then 10.0 mmol acetyl aromatic compound was added dropwise. The mixture was stirred at room temperaturefor2-8 h(36-54 hfor2nand2oduetothesteric hindrance of thenaphthyl andbiphenyl). Theend ofthereaction was monitored by TLC. Afterward, water (20 mL) and EtOAc (75 mL, 25 mL × 3) were added into the mixture. Theinorganic precipitatewas removed, and theorganic solution was washed with brine three times and dried. The crude product was puried by silica gel column chromatography with EtOAc/hexane as eluent to get pureα,β-unsaturated carbonyl compounds (2a-2o).
References:
Zhan, Xiaoping;Lan, Lan;Zhang, Yuankui;Chen, Jian;Zhao, Kai;Wang, Shuai;Xin, Yuxuan;Mao, Zhenmin [Bulletin of the Korean Chemical Society,2016,vol. 37,# 2,p. 200 - 206]

90956-78-8
0 suppliers
inquiry

6552-63-2
46 suppliers
$21.00/250mg
104-46-1
331 suppliers
$5.00/100mg

6552-63-2
46 suppliers
$21.00/250mg
104-88-1
646 suppliers
$5.00/25g
100-06-1
606 suppliers
$22.10/25g

6552-63-2
46 suppliers
$21.00/250mg
![Benzene, 1-chloro-4-[1-[(trimethylsilyl)oxy]ethenyl]-](/CAS/20200401/GIF/58518-76-6.gif)
58518-76-6
1 suppliers
inquiry

123-11-5
881 suppliers
$10.00/10g

6552-63-2
46 suppliers
$21.00/250mg