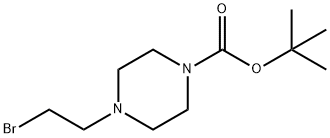
4-(2-BROMOETHYL)-1-PIPERAZINECARBOXYLIC ACID, 1,1-DIMETHYLETHYL ESTER synthesis
- Product Name:4-(2-BROMOETHYL)-1-PIPERAZINECARBOXYLIC ACID, 1,1-DIMETHYLETHYL ESTER
- CAS Number:655225-01-7
- Molecular formula:C11H21BrN2O2
- Molecular Weight:293.2
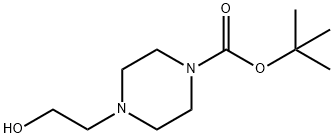
77279-24-4

655225-01-7
General procedure for the synthesis of N-BOC-4-bromoethylpiperazine from tert-butyl 4-(2-hydroxyethyl)piperazine-1-carboxylate: carbon tetrabromide (1.681 g, 5.07 mmol) was dissolved in dichloromethane (17 ml) and cooled in an ice bath. Subsequently, tert-butyl 4-(2-hydroxyethyl)piperazine-1-carboxylate (1.062 g, 4.61 mmol) and triphenylphosphine (1.329 g, 5.07 mmol) were added to this solution. The reaction mixture was stirred overnight at room temperature and additional dichloromethane (11 ml) was added. After completion of the reaction, the solvent was removed by evaporation. The residue was purified by silica gel column chromatography (using a SNAP ULTRA 25g column with hexane/ethyl acetate solvent mixture as eluent) to afford N-BOC-4-bromoethylpiperazine (1.131 g, 83.7% yield) as a yellow solid.

77279-24-4
148 suppliers
$15.00/500mg

655225-01-7
77 suppliers
$26.00/100mg
Yield: 83.7%
Reaction Conditions:
with carbon tetrabromide;triphenylphosphine in dichloromethane at 20;Cooling with ice;
Steps:
168.168-1 Example 168-1: Synthesis of tert-butyl 4-(2-bromomethyl)piperazine-1-carboxylate
Carbon tetrabromide (1.681 g, 5.07 mmol) was dissolved in dichloromethane (17 ml), cooled with ice.thereTertiary butyl 4- (2-hydroxyMethyl) piperazine-1-carboxylate (1.062 g, 4.61 mmol), triphenylphosphine (1.329 g, 5.07 mmol), and stirred overnight at room temperature was added dichloromethane (11 ml). After the reaction, the solvent was evaporated. The residue was purified by silica gel column chromatography (SNAP ULTRA 25 g, hexane / ethyl acetate) to give the title compound (1.131 g, 83.7%) as a yellow solid.
References:
Yakult Honsha Co., Ltd.;University of Occupational and Environmental Health;Ono, Masahiro;Kobayashi, Tsuneyuki;Yamazaki, Ryuta;Haibara, Hirotake;Nishiyama, Yukiko;Hokkyo, Atsuko;Nishiyama, Hiroyuki;Kurita, Akinobu;Matsuzaki, Ken;Kono, Kimitoshi;Izumi, Hiroto JP2016/124812, 2016, A Location in patent:Paragraph 0367

106-93-4
405 suppliers
$15.00/5g
57260-71-6
727 suppliers
$5.00/5g

655225-01-7
77 suppliers
$26.00/100mg

57260-71-6
727 suppliers
$5.00/5g

655225-01-7
77 suppliers
$26.00/100mg

24424-99-5
868 suppliers
$13.50/25G

655225-01-7
77 suppliers
$26.00/100mg
103-76-4
532 suppliers
$6.00/25g

655225-01-7
77 suppliers
$26.00/100mg