
7-bromo-2,3,4,5-tetrahydro-1H-benzo[c]azepine synthesis
- Product Name:7-bromo-2,3,4,5-tetrahydro-1H-benzo[c]azepine
- CAS Number:740842-87-9
- Molecular formula:C10H12BrN
- Molecular Weight:226.11
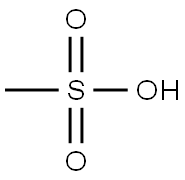
75-75-2
651 suppliers
$10.00/5g
4133-35-1
225 suppliers
$50.00/5g
![7-bromo-2,3,4,5-tetrahydro-1H-benzo[c]azepine](/CAS/20180703/GIF/740842-87-9.gif)
740842-87-9
5 suppliers
inquiry
![7-BROMO-2,3,4,5-TETRAHYDRO-1H-BENZO[D]AZEPINE](/StructureFile/ChemBookStructure21/GIF/CB7819805.gif)
740842-86-8
9 suppliers
inquiry
Yield:740842-86-8 18.9% ,740842-87-9 11%
Reaction Conditions:
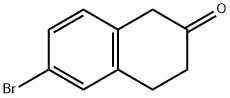
Stage #1: methanesulfonic acid;6-bromo-2-tetralonewith sodium azide at 0 - 20; for 5 h;Inert atmosphere;
Stage #2: with dimethylsulfide borane complex at 0; for 7 h;Inert atmosphere;Reflux;
Steps:
Step 1. .7-bromo-1,3,4,5-tetrahydro-2H-benzo[d]azepin-2-one and 7-bromo-1,2,4,5-tetrahydro-3H-benzo[c]azepin-3-one (9c and 10c)
To a 500mL round-bottom flask was added 6-bromo-2-tetralone (10g, 44.43mmol) and methanesulfonic acid (47mL, 72mmol) at 0°C. Sodium azide (3.61g, 55.5mmol) was slowly added to the solution and the reaction was stirred at room temperature for 5h. The reaction mixture was slowly poured into a 2000-mL beaker containing potassium hydroxide (49.8g, 888mmol) in water (800mL) with vigorous stirring. After the acid was completely quenched, the aqueous solution was extracted with EtOAc (3×500mL). The combined organic layers were washed with saturated aqueous NaHCO3 (100mL) and brine (100mL) respectively, dried over anhydrous MgSO4 and concentrated to give a mixture of 7-bromo-1,3,4,5-tetrahydro-2H-benzazepin-2-one and 7-bromo-1,2,4,5-tetrahydro-3H-benzazepin-3-one (9c and 10c) as brown solid which was used without purification. Step 2.7-bromo-2,3,4,5-tetrahydro-1H-benzo[d]azepine and 7-bromo-2,3,4,5- tetrahydro -1H-benzo[c]azepine (9a and 10a): To a 1000mL round-bottom flask was added the mixture of 7-bromo-1,3,4,5-tetrahydro-2H-benzazepin-2-one and 7-bromo-1,2,4,5-tetrahydro-3H-benzazepin-3-one (9c and 10c) (13g, 54.2mmol) and tetrahydrofuran (50mL). The solution was cooled to 0°C and borane-methylsulfide complex (50mL, 100M) was slowly added. The reaction mixthre was stirred at 0°C for 10min, warmed to room temperature and stirred for 3h, then heated at reflux for 4h. Upon cooling to room temperature, the mixture was quenched with MeOH (50mL) to romove excess borane. The solvent was concentrated under reduced pressure. The residue was purified by for 2h. The solution was then filtered and the filtrate was concentrated under reduced pressure. The residue was purified by chromatography (PE:EA=10:1) and reverse-phase chromatography to give 7-bromo-2,3,4,5-tetrahydro-1H-benzoazepine (9a, 1.9g, 18.9%) and 7-bromo-2,3,4,5-tetrahydro-1H-benzoazepineand (10a, 1.1g, 11.0%), Both of 9a and 10a were colorless oil. 9a: 1H NMR (400MHz, CDCl3) δ 7.30 (d, J=10.4Hz, 2H), 7.00 (d, J=7.8Hz, 1H), 4.20 (s, 1H), 3.62-3.40 (m, 2H), 3.12-2.73 (m, 4H), 2.68 (dd, J=21.2, 9.2Hz, 2H); LC-MS (ESI): tR=5.175min, m/z=226.0, 228.0 [M+H]+. 10a: 1H NMR (400MHz, CDCl3) δ 7.33 (d, J=8.1Hz, 2H), 7.09 (d, J=7.7Hz, 1H), 4.13 (d, J=14.7Hz, 1H), 3.99 (dd, J=14.7, 9.7Hz, 1H), 3.58 (d, J=14.1Hz, 1H), 3.43 (s, 1H), 3.10-2.92 (m, 2H), 2.83 (dd, J=14.8, 6.7Hz, 1H), 2.16-2.05 (m, 1H), 1.73-1.64 (m, 1H); LC-MS (ESI): tR=5.096 min, m/z=226.0, 228.0 [M+H]+.
References:
Sun, Nannan;Ma, Xiaojun;Zhou, Kaifeng;Zhu, Chen;Cao, Zhonglian;Wang, Yonghui;Xu, Jun;Fu, Wei [European Journal of Medicinal Chemistry,2020,vol. 187,art. no. 111984]
![7-bromo-4,5-dihydro-1H-benzo[c]azepin-3(2H)-one](/CAS/20180629/GIF/740842-85-7.gif)
740842-85-7
4 suppliers
inquiry
![7-bromo-2,3,4,5-tetrahydro-1H-benzo[c]azepine](/CAS/20180703/GIF/740842-87-9.gif)
740842-87-9
5 suppliers
inquiry

4133-35-1
225 suppliers
$50.00/5g
![7-bromo-2,3,4,5-tetrahydro-1H-benzo[c]azepine](/CAS/20180703/GIF/740842-87-9.gif)
740842-87-9
5 suppliers
inquiry