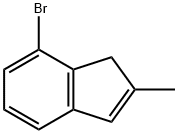
7-broMo-2-Methyl-1H-Indene synthesis
- Product Name:7-broMo-2-Methyl-1H-Indene
- CAS Number:880652-93-7
- Molecular formula:C10H9Br
- Molecular Weight:209.08
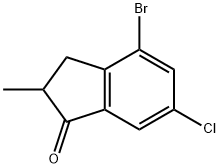
869063-68-3

880652-93-7
7-Bromo-2-methyl-1H-indene was synthesized as follows: to a solution of 116 g (0.52 mol) 4-bromo-6-chloro-2-methyl-2,3-dihydro-1H-inden-1-one in 950 mL THF-methanol (2:1, v/v) was added in batches of 38.3 g (1.02 mol) NaBH4 at -5 °C, and the addition process lasted for 2 h. (Note : the temperature must be kept below 0°C). After completion of the addition, the reaction mixture was stirred at room temperature overnight. Subsequently, the reaction mixture was poured into 1000 mL of ice water and acidified with 10% HCl to pH=4. The organic layer was separated and the aqueous layer was extracted with methyl tert-butyl ether (3 x 300 mL). The organic phases were combined, dried with K2CO3 and concentrated to dryness under reduced pressure. To the residue, 1500 mL of toluene was added and about 2 g of p-toluenesulfonic acid (p-TolSO3H) was added as a catalyst and the reaction was refluxed for 2 hours. After completion of the reaction, the mixture was cooled to room temperature, rapidly filtered through a short silica gel 60 column (40-63 μm, 60 mm diameter, 140 mm height) and the column was washed with 250 mL of toluene. The filtrate and washings were combined and concentrated to dryness under reduced pressure. Purification by fractional distillation afforded the target product 7-bromo-2-methyl-1H-indene, boiling point 104-108 °C/5 mmHg, yield 100 g (93%) as a colorless solid. Elemental analysis Calculated values (C10H9Br): C, 57.44; H, 4.34. Measured values: C, 57.59; H, 4.40. 1H NMR (CDCl3) δ: 7.23 (dd, J = 7.9 Hz, J = 1.0 Hz, 1H, 6-H), 7.18 (dd, J = 7.4 Hz, J = 1.0 Hz, 1H, 4-H), 7.10 (m, 1H, 4-H). 7.10 (m, 1H, 5-H), 6.51 (m, 1H, 3-H), 3.28 (m, 2H, 1,1'-H), 2.17 (s, 3H, 2-Me).13C NMR (CDCl3) δ: 147.3, 146.8, 143.3, 128.2, 127.1, 126.6, 118.7, 118.3. 44.2, 16.7.

869063-68-3
9 suppliers
$527.00/5g

880652-93-7
74 suppliers
$10.00/250mg
Yield: 100 g (93%)
Reaction Conditions:
with sodium tetrahydroborate;pTolSO3H in THF-methanol;toluene
Steps:
3 7-Bromo-2-methyl-1H-indene
7-Bromo-2-methyl-1H-indene To a solution of 116 g (0.52 mol) of 4-bromo-6-chloro-2-methyl-1-indanone in 950 ml of THF-methanol (2:1, vol.), 38.3 g (1.02 mol) of NaBH4 were added in small portions for 2 h at -5° C. (Caution: temperature must be lower 0° C.). The mixture was stirred overnight at ambient temperature. The resulting mixture was poured on 1000 cm3 of ice and acidified with 10% HCl to pH=4. The organic layer was separated and the aqueous layer was extracted with 3*300 ml of methyl-tert-butyl ether. This combined extract was dried over K2CO3 and evaporated to dryness. To the residue 1500 ml of toluene were added and the resulting toluene solution was treated with catalytic amount of pTolSO3H (ca. 2 g) for 2 h at reflux. Then this mixture was cooled to room temperature and passed through a short Silica Gel 60 column (40-63 μm, d 60 mm, 1 40 mm). This column was additionally eluted with 250 ml of toluene. The combined extract was evaporated to dryness. Fractional distillation gave a mixture of the title indenes, b.p. 104-108° C./5 mm Hg. Yield 100 g (93%) of colorless solid. Anal. calc. for C10H9Br: C, 57.44; H, 4.34. Found: C, 57.59; H, 4.40. 1H NMR (CDCl3): δ 7.23 (dd, J=7.9 Hz, J=1.0 Hz, 1H, 6-H), 7.18 (dd, J=7.4 Hz, J=1.0 Hz, 1H, 4-H), 7.10 (m, 1H, 5-H), 6.51 (m, 1H, 3-H), 3.28 (m, 2H, 1,1'-H), 2.17 (s, 3H, 2-Me). 13C NMR (CDCl3): δ 147.3, 146.8, 143.3, 128.2, 127.1, 126.6, 118.7, 118.3,44.2,16.7.
References:
Voskoboynikov, Alexander Z.;Ryabov, Alexey N.;Nikulin, Mikhail V.;Lygin, Alexander V.;Coker, Catalina L.;Canich, Jo Ann M. US2007/135595, 2007, A1
174702-59-1
88 suppliers
$45.00/1 g

880652-93-7
74 suppliers
$10.00/250mg