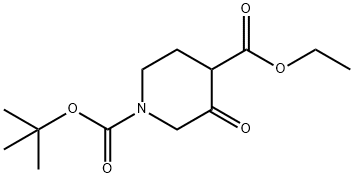
3-Oxo-Piperidine-1,4-Dicarboxylic Acid 1-Tert-Butyl Ester 4-Ethyl Ester synthesis
- Product Name:3-Oxo-Piperidine-1,4-Dicarboxylic Acid 1-Tert-Butyl Ester 4-Ethyl Ester
- CAS Number:71233-25-5
- Molecular formula:C13H21NO5
- Molecular Weight:271.31

24424-99-5
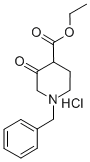
52763-21-0

71233-25-5
1. A Parr autoclave was charged with ethyl 1-benzyl-3-oxopiperidine-4-carboxylate hydrochloride (10.0 g, 33.58 mmol), 10% Pd/C catalyst (1.0 g), di-tert-butyl dicarbonate (Boc2O, 14.64 g, 67.16 mmol), sodium carbonate (Na2CO3, 3.56 g, 33.58 mmol) and anhydrous ethanol (EtOH, 100 ml). 2. Hydrogen was introduced into the autoclave to a pressure of 38 bar and the reaction mixture was subsequently stirred at 50 °C for 48 h. After the reaction was completed, the reaction mixture was purified by boiling the hydrogen gas under high pressure. 3. After completion of the reaction, the autoclave was cooled to 25 °C and the hydrogen pressure was slowly released. 4. The catalyst was removed by filtration and the filtrate was concentrated under reduced pressure to give a yellow oil. 5. The crude product was purified by column chromatography (eluent: hexane/ethyl acetate, 2:1) to afford ethyl 1-N-Boc-3-oxopiperidine-4-carboxylate (9.10 g, 100% yield) as a clear oil. 6. The product was subjected to thin layer chromatography (TLC). 6. The product was detected by thin layer chromatography (TLC) with an Rf value of 0.70 (Expanding agent: hexane/ethyl acetate, 2:1). 7. The structure of the product was confirmed by 1H NMR (CDCl3, 400 MHz) and HRMS (CI) and the results were as expected.

24424-99-5
868 suppliers
$13.50/25G

52763-21-0
242 suppliers
$6.00/1g

71233-25-5
151 suppliers
$6.00/1g
Yield:71233-25-5 100%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen;sodium carbonate in ethanol at 50; under 28502.9 Torr; for 48 h;Autoclave;
Steps:
64 1-tert-butyl 4-ethyl3-oxopiperidine-1,4-dicarboxylate (74)
1-tert-butyl 4-ethyl3-oxopiperidine-1,4-dicarboxylate (74)
A mixture of ethyl 1-benzyl-3-oxo-4-piperidinecarboxylate hydrochloride (10.0 g,33.58 mmol), Pd/C (10% wt., 1.0 g), Boc20 (14.64g, 67.16 mmol), Na2C03 (3.56 g,33.58 mmol) and EtOH (100 ml)was loaded into a Parr autoclave. Hydrogen was10 introduced (38 bar), and the mixturewas stirred at 50 oc for 48 h. Afterthe autoclave was cooled to 25 oc the hydrogen pressurewas released, the catalyst was removed by filtration, and the mixture was concentrated under reduced pressureto give a yellow oil. Purification by column chromatography (hexane: EtOAc 2:1) gave the title compound(9.10 g, 100%) as a clear oil.15Rf = 0.70 (hexane:EtOAc 2:1); 1H NMR (CDCI3,400 MHz) o 12.10 (s, 1H, enolform -OH),4.25 (q, J= 7.1 Hz, 2H), 4.04 (s, 2H), 3.50 (t, J= 5.8 Hz, 2H), 2.33 (t, J= 5.8 Hz, 2H),1.48 (s, 9H), 1.32 (t, J = 7.1 Hz, 3H); HRMS (CI) calc. for C13H21NOs (M+NH4)+ 289.1763, found: 289.1759.
References:
CANCER RESEARCH TECHNOLOGY LIMITED;IMPERIAL INNOVATIONS LIMITED;BONDKE, Alexander;KROLL, Sebastian;BARRETT, Anthony;FUCHTER, Matthew;SLAFER, Brian;ALI, Simak;COOMBES, Charles;SNYDER, James Patrick WO2015/124941, 2015, A1 Location in patent:Page/Page column 134

24424-99-5
868 suppliers
$13.50/25G
39514-19-7
136 suppliers
$17.00/1g

71233-25-5
151 suppliers
$6.00/1g

24424-99-5
868 suppliers
$13.50/25G
70637-75-1
35 suppliers
$90.00/50mg

71233-25-5
151 suppliers
$6.00/1g

52763-21-0
242 suppliers
$6.00/1g

71233-25-5
151 suppliers
$6.00/1g

39514-19-7
136 suppliers
$17.00/1g

71233-25-5
151 suppliers
$6.00/1g