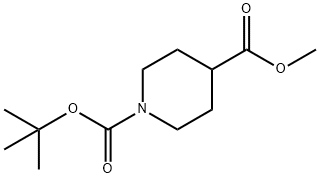
N-Boc-Piperidine-4-carboxylic acid methyl ester synthesis
- Product Name:N-Boc-Piperidine-4-carboxylic acid methyl ester
- CAS Number:124443-68-1
- Molecular formula:C12H21NO4
- Molecular Weight:243.3

67-56-1
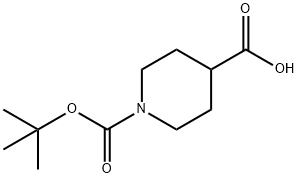
84358-13-4

124443-68-1
General procedure for the synthesis of methyl 1-tert-butoxycarbonyl-4-piperidinecarboxylate from methanol and 1-Boc-4-piperidinecarboxylic acid: a hexane solution of trimethylmethylsilyl diazomethane (2.00 M, 52.9 mL, 106 mmol) was added slowly and dropwise at 0 °C to a 1-tert-butoxycarbonyl-piperidine-4-carboxylic acid (12.14 g, 52.95 mmol) acetonitrile ( 100 mL) suspension. Methanol (10 mL) was then added. The reaction mixture was allowed to stand at 0 °C for 30 min and then stirred at room temperature for 3 h. The reaction was carried out under reduced pressure. Upon completion of the reaction, the solvent was removed by distillation under reduced pressure. The residue was purified by column chromatography [hexane/ethyl acetate (4.5:1, v/v)] to afford 1-tert-butyl 4-methylpiperidine-1,4-dicarboxylate as a colorless oil (11.5 g, 90% yield). The structure of the product was confirmed by 1H NMR (400 MHz, d6-DMSO): δ 4.02 (dt, 2H, J = 3.5, 13.7 Hz), 3.69 (s, 3H), 2.82 (ddd, 2H, J = 3, 11.5, 14.5 Hz), 2.45 (tt, 1H, J = 3.9, 11.5 Hz), 1.90-1.84 ( m, 2H), 1.67-1.57 (m, 2H), 1.45 (s, 9H). Mass spectral analysis (ES) showed m/z 265.8 (MNa+); calculated value: 243.1 (M).
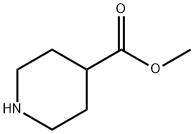
2971-79-1
271 suppliers
$10.00/10g

24424-99-5
871 suppliers
$13.50/25G

124443-68-1
246 suppliers
$6.00/5g
Yield:124443-68-1 98%
Reaction Conditions:
with triethylamine in dichloromethane at 25; for 12 h;Product distribution / selectivity;
Steps:
A.2.1
Synthesis of 9-(Azetidin-1-yl)-9-phenyl-3-azaspiro[5.5]undecane Trifluoroacetate (A4); Step-1: 1-tert-Butyl 4-methyl piperidine-1,4-dicarboxylate; To a stirred solution of methyl isonipecotate (127.2 mmol, 1 eq) in dichloromethane (200 ml) were added di-tert-butyldicarbonate (190.8 mmol, 1.5 eq) and triethylamine (254.4 mmol, 2.0 eq). The reaction mixture was stirred for 12 h at 25° C. It was then diluted with dichloromethane (100 ml) and washed with water (50 ml) and brine (50 ml). The organic layer was separated, dried over Na2SO4, concentrated and employed in the next step without further purification. Yield: 98%
References:
US2010/113417,2010,A1 Location in patent:Page/Page column 36

67-56-1
790 suppliers
$9.00/25ml

84358-13-4
379 suppliers
$5.00/5g

124443-68-1
246 suppliers
$6.00/5g

67-56-1
790 suppliers
$9.00/25ml
498-94-2
0 suppliers
$5.00/10g

24424-99-5
871 suppliers
$13.50/25G

124443-68-1
246 suppliers
$6.00/5g

84358-13-4
379 suppliers
$5.00/5g

74-88-4
357 suppliers
$15.00/10g

124443-68-1
246 suppliers
$6.00/5g

84358-13-4
379 suppliers
$5.00/5g
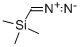
18107-18-1
210 suppliers
$33.00/5g

124443-68-1
246 suppliers
$6.00/5g