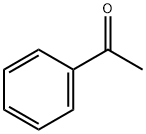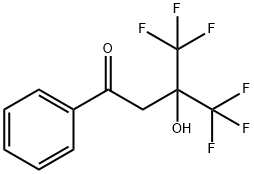
4,4,4-TRIFLUORO-3-HYDROXY-3-(TRIFLUOROMETHYL)BUTYROPHENONE, 97% MIN. synthesis
- Product Name:4,4,4-TRIFLUORO-3-HYDROXY-3-(TRIFLUOROMETHYL)BUTYROPHENONE, 97% MIN.
- CAS Number:731-00-0
- Molecular formula:C11H8F6O2
- Molecular Weight:286.17
Yield:731-00-0 95.1 %Chromat.
Reaction Conditions:
with sulfuric acid at 50 - 60; under 750.075 - 3750.38 Torr; for 7 h;
Steps:
11
A 100 mL pressure-proof glass reactor equipped with a pressure gauge, a thermometer and a gas introducing tube was charged with a stirring magnet coated with tetrafluoroethylene resin, 17.3 g (0.14 moles) of acetophenone and 0.04 g of concentrated sulfuric acid, followed by introducing 20.0 g (0.12 moles) of 1,1,1,3,3,3-hexafluoroacetone with stirring using a stirrer at a temperature range of 50 to 60° C. by spending 2 hr. Upon this, the pressure was 0.5 MPa. After introducing 1,1,1,3,3,3-hexafluoroacetone, the reaction liquid was stirred at a temperature of 50 to 60° C. for 5 hr. When the pressure lowered to 0.1 MPa, the reaction was terminated. As a result, 35.0 g of a reaction mixture were obtained. The reaction liquid, except excessive acetophenone, was found by gas chromatography to contain 95.1% of the target 4,4,4-trifluoro-3-hydroxy-1-phenyl-3-(trifluoromethyl)butan-1-one and 4.9% of impurities. 35.0 g of the obtained reaction mixture were subjected to a vacuum distillation under 2.9 kPa, thereby collecting a distillate having a boiling point range of 135 to 137° C. With this, there were obtained 28.1 g of the target 4,4,4-trifluoro-3-hydroxy-1-phenyl-3-(trifluoromethyl)butan-1-one (purity: 98.0%). The yield was 80.2%. The NMR data of the target product are as follows. 1H NMR (solvent: CDCl3; standard substance: TMS); δ 7.94-7.97 (m, 2H), 7.66-7.71 (m, 1H), 7.50-7.55 (m, 1H), 7.21 (s, 1H), 3.46 (s, 2H) 19F NMR (solvent: CDCl3; standard substance: CCl3F); δ -78.5 (s, 6F).
References:
US2005/215836,2005,A1 Location in patent:Page/Page column 9
13735-81-4
115 suppliers
$23.52/1g
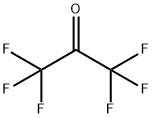
684-16-2
0 suppliers
$120.00/5g

731-00-0
11 suppliers
$45.00/50mg
![1-Butanone, 4,4,4-trifluoro-1-phenyl-3-(trifluoromethyl)-3-[(trimethylsilyl)oxy]-](/CAS/20210305/GIF/143211-22-7.gif)
143211-22-7
0 suppliers
inquiry

731-00-0
11 suppliers
$45.00/50mg

13735-81-4
115 suppliers
$23.52/1g

731-00-0
11 suppliers
$45.00/50mg