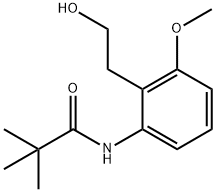
N-[2-(2-hydroxyethyl)-3-Methoxyphenyl]-2,2- diMethylpropanaMide synthesis
- Product Name:N-[2-(2-hydroxyethyl)-3-Methoxyphenyl]-2,2- diMethylpropanaMide
- CAS Number:76093-72-6
- Molecular formula:C14H21NO3
- Molecular Weight:251.32
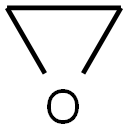
75-21-8
239 suppliers
$39.10/1mL
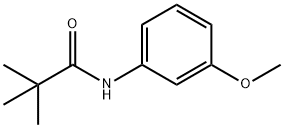
56619-93-3
90 suppliers
$22.00/1g
![N-[2-(2-hydroxyethyl)-3-Methoxyphenyl]-2,2-
diMethylpropanaMide](/CAS/GIF/76093-72-6.gif)
76093-72-6
14 suppliers
$354.80/250mg
Yield:76093-72-6 77.1%
Reaction Conditions:
Stage #1: N-(3-methoxyphenyl)pivalamidewith n-butyllithium in tetrahydrofuran at 0; for 2 h;Inert atmosphere;
Stage #2: oxirane in tetrahydrofuran;diethyl ether at 0 - 20; for 3 h;
Steps:
1 Synthesis of N-[2-(2-hydroxyethyl)-3-methoxyphenyl]pivalamide (1b)
Synthesis of N-[2-(2-hydroxyethyl)-3-methoxyphenyl]pivalamide (1b)
Under the argon atmosphere, n-butyllithium (nBuLi) (2.6 M in THF, 111 mL, 289 mmol, commercial product) was slowly dropped at 0°C into a tetrahydrofuran (THF) (400 mL, dehydrated, commercial product) solution of the compound 1a (30.0 g, 145 mmol).
After the mixture was stirred at 0°C for 2 hours, ethylene oxide (1.3 M ether solution, 175 mL, 228 mmol, commercial product) was slowly added to the mixture and stirred at 0°C for 1 hour.
The temperature was raised to room temperature, and then the mixture was further stirred for 2 hours.
The mixture was concentrated under reduced pressure, to which a saturated ammonium chloride aqueous solution (sat. NH4Cl aq.) was added.
Subsequently, the mixture was extracted with ethyl acetate (EtOAc) (100 mL * 4).
The combined organic layer was dried over sodium sulfate and filtered.
The filtrate was concentrated under reduced pressure.
The residue was recrystallized with ethyl acetate (EtOAc), and thus N-[2-(2-hydroxyethyl)-3-methoxyphenyl]pivalamide (compound 1b) (28.1 g, 112 mmol, 77.1%) was obtained as a colorless solid.
TLC Rf= 0.40 (n-hexane/EtOAc = 3/1)
References:
EP2881397,2015,A1 Location in patent:Paragraph 0053; 0056; 0057

75-21-8
239 suppliers
$39.10/1mL
![N-[2-(2-hydroxyethyl)-3-Methoxyphenyl]-2,2-
diMethylpropanaMide](/CAS/GIF/76093-72-6.gif)
76093-72-6
14 suppliers
$354.80/250mg
3282-30-2
380 suppliers
$22.00/100ml
![N-[2-(2-hydroxyethyl)-3-Methoxyphenyl]-2,2-
diMethylpropanaMide](/CAS/GIF/76093-72-6.gif)
76093-72-6
14 suppliers
$354.80/250mg
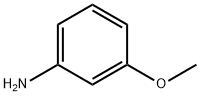
536-90-3
298 suppliers
$10.00/5g
![N-[2-(2-hydroxyethyl)-3-Methoxyphenyl]-2,2-
diMethylpropanaMide](/CAS/GIF/76093-72-6.gif)
76093-72-6
14 suppliers
$354.80/250mg

3282-30-2
380 suppliers
$22.00/100ml

536-90-3
298 suppliers
$10.00/5g
![N-[2-(2-hydroxyethyl)-3-Methoxyphenyl]-2,2-
diMethylpropanaMide](/CAS/GIF/76093-72-6.gif)
76093-72-6
14 suppliers
$354.80/250mg