
7H-Furo[3,2-g][1]benzopyran-7-one, 3-methyl- synthesis
- Product Name:7H-Furo[3,2-g][1]benzopyran-7-one, 3-methyl-
- CAS Number:1137-75-3
- Molecular formula:C12H8O3
- Molecular Weight:200.19
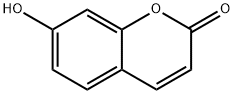
93-35-6
512 suppliers
$6.00/100mg
584-08-7
1245 suppliers
$5.00/100g
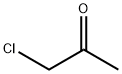
78-95-5
0 suppliers
$21.50/5G
![7H-Furo[3,2-g][1]benzopyran-7-one, 3-methyl-](/CAS/20180601/GIF/1137-75-3.gif)
1137-75-3
3 suppliers
inquiry
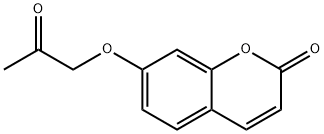
36914-75-7
12 suppliers
$75.00/100mg
Yield:36914-75-7 82%
Reaction Conditions:
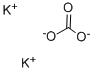
with potassium iodide in acetone;
Steps:
5'-AMINOMETHYL-4'-METHYLPSORALEN
A mixture of potassium iodide (1.0 g, 6 mmol), chloroacetone (28.43 mL, 33.03 g, 0.357 mole) and reagent grade acetone (400 mL., dried over K2 CO3) was allowed to stand overnight. 7-Hydroxycoumarin (50.0 g, 0.308 mol), anhyd. K2 CO3 (49.34 g, 0.357 mole), and dry reagent grade acetone (1 L) were added and the mixture was refluxed for about 24 hours with overhead stirring and protection from atmospheric moisture (Drierite-TM tube). The hot reaction mixture was filtered and the precipitate was washed with two portions (200 mL) of dry reagent grade acetone. The filtrate and washes were combined and evaporated in vacuo to obtain a first crop. A mixture of the precipitate and water (1 L) was extracted with two portions (700 mL) of trichloromethane, which were combined, dried (MgSO4), and evaporated in vacuo to obtain more product. A trichloromethane (CHCl3) solution (3 L) of the two crops was washed once with 5% aqueous NaOH (1 L), and then three times with water (1 L), dried (MgSO4), and evaporated, in vacuo to obtain crude 7-acetonyloxycoumarin (64.65 g, 96%), mp 168.2°-169.9° C., which was suitable for use in the next step. Recrystallization of a portion from 95% ethanol gave a purer sample (85.3% recovery, 82% yield), mp 174.6°-174.8° C. (previous run: mp 166.9°-167.1° C.) The NMR (CDCl3) spectrum was identical to that obtained in the previous run. 4'-Methylpsoralen.
References:
US4370344,1983,A

36914-75-7
12 suppliers
$75.00/100mg
![7H-Furo[3,2-g][1]benzopyran-7-one, 3-methyl-](/CAS/20180601/GIF/1137-75-3.gif)
1137-75-3
3 suppliers
inquiry

93-35-6
512 suppliers
$6.00/100mg
![7H-Furo[3,2-g][1]benzopyran-7-one, 3-methyl-](/CAS/20180601/GIF/1137-75-3.gif)
1137-75-3
3 suppliers
inquiry
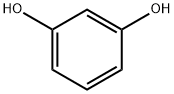
108-46-3
791 suppliers
$10.00/10g
![7H-Furo[3,2-g][1]benzopyran-7-one, 3-methyl-](/CAS/20180601/GIF/1137-75-3.gif)
1137-75-3
3 suppliers
inquiry