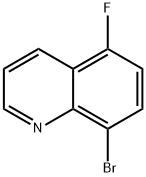
8-bromo-5-fluoroquinoline synthesis
- Product Name:8-bromo-5-fluoroquinoline
- CAS Number:917251-99-1
- Molecular formula:C9H5BrFN
- Molecular Weight:226.05
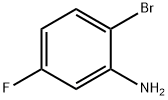
1003-99-2

56-81-5

917251-99-1
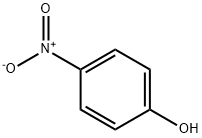
2-Bromo-5-fluoroaniline (100 g, 1.0 eq.), 4-nitrophenol (40 g, 0.54 eq.) and glycerol (97 g, 2.0 eq.) were slowly added to pre-mixed sulfuric acid (267 mL) and water (114 mL) at 140-150 °C and the reaction lasted for 1.5 h. The reaction was carried out by HPLC. At the beginning of the reaction, the relative area percentage of 4-nitrophenol was shown to be 37.8% by HPLC analysis. The content of 4-nitrophenol decreased to 4.7% after the addition of 50% of the feedstock and further decreased to 5.0% after the addition of all the feedstock. Upon completion of the reaction, 8-bromo-5-fluoroquinoline was obtained after post-treatment in 87.5% yield with 0.29% total impurities. In addition, reducing the amount of 4-nitrophenol to 0.46 equivalents (34 g) was also successful in synthesizing the target intermediate in acceptable yield.

1003-99-2
315 suppliers
$6.00/5g

56-81-5
1770 suppliers
$5.00/25g

917251-99-1
80 suppliers
$60.00/250mg
Yield: 89.8%
Reaction Conditions:
Stage #1:2-bromo-5-fluoroaniline;glycerol with sulfuric acid;sodium 3-nitrobenzenesulfonate in water at 60 - 130; for 3 h;
Stage #2: with sodium hydroxide in tert-butyl methyl ether;waterProduct distribution / selectivity;
Steps:
DD
1-(5-fluoroquinolin-8-yl)piperidin-4-one (Example P, Step 3) is also prepared as shown in the reaction scheme above. To a 500 ml flask equipped with a stirrer, a thermocouple, a condenser and nitrogen inlet were charged 50 g 2-bromo-5-fluoroaniline and 60 g glycerol. The mixture was heated to 60° C. and 55 g nitrobenzene sulfonic acid was charged in portion. The mixture was then heated to 100-110° C., started charge 200 ml 70% sulfuric acid. After sulfuric acid addition, the mixture was heat to 130° C. and stirred for 3 hours before cooled to room temperature. Water (300 g) was added and a grayish by product was filtered off. The filtrate was slowly added into a 2-L reactor containing a mix of 420 g 50% NaOH, 420 g of water, 352 g methyl tert-butyl ether. After filtering off small amount solid, the aqueous layer was split off and the organic layer was washed with 10% NaOH (2×100 ml), 20% NaCl (2×200 ml), the solvent was removed, weight 56 g, 89.8%.
References:
Wyeth US2007/27160, 2007, A1 Location in patent:Page/Page column 66
100-02-7
61 suppliers
$11.00/5G

1003-99-2
315 suppliers
$6.00/5g

917251-99-1
80 suppliers
$60.00/250mg

56-81-5
1770 suppliers
$5.00/25g

917251-99-1
80 suppliers
$60.00/250mg