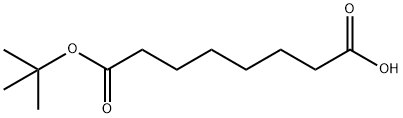
8-(tert-Butoxy)-8-oxooctanoic acid synthesis
- Product Name:8-(tert-Butoxy)-8-oxooctanoic acid
- CAS Number:234081-94-8
- Molecular formula:C12H22O4
- Molecular Weight:230.3
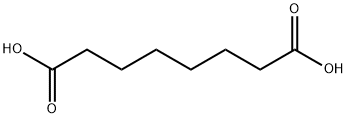
505-48-6

75-65-0

234081-94-8
The general procedure for the synthesis of 8-(tert-butoxy)-8-oxooctanoic acid from octanedioic acid and tert-butanol was as follows: octanedioic acid (1.00 g, 5.75 mmol), 2-methyl-2-propanol (6.9 mL, 71.8 mmol), EDCI (1.1 g, 5.74 mmol) and DMAP (0.7 g, 5.74 mmol) were dissolved in dichloromethane ( 6.8 mL). The reaction mixture was stirred at room temperature for 5 hours. After completion of the reaction, the reaction mixture was diluted with ether (60 mL) and washed sequentially with 0.01 N HCl (50 mL) and water (50 mL). The organic phase was dried with anhydrous magnesium sulfate and subsequently concentrated under reduced pressure to remove the solvent. The crude product was purified by fast column chromatography with the eluent being 1:1 ethyl acetate:petroleum ether to afford 8-(tert-butoxy)-8-oxooctanoic acid (0.41 g, 1.77 mmol, 31% yield) as a colorless oil.1H NMR (400 MHz, DMSO-d6) δ ppm: 11.94 (broad single peak, 1H), 2.18 (double peak, J = 14.6, 7.3 Hz, 4H), 1.54-1.42 (multiple peaks, 4H), 1.40 (single peak, 9H), 1.34-1.20 (multiple peaks, 4H).

505-48-6
447 suppliers
$8.00/10g

75-65-0
784 suppliers
$10.00/10ml

234081-94-8
60 suppliers
$35.00/250mg
Yield: 31%
Reaction Conditions:
with dmap;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in dichloromethane at 20; for 5 h;
Steps:
25 Preparation 25: 8-(ferf-Butoxy)-8-oxooctanoic acid
Octanedioic acid (1.00 g, 5.75 mmol), 2-methyl-2-propanol (6.9 mL, 71.8 mmol), EDCI (1.1 g, 5.74 mmol) and DMAP (0.7 g, 5.74 mmol) were dissolved in DCM (6.8 mL). The reaction was stirred at room temperature for 5 hours. The reaction mixture was diluted with diethyl ether (60 mL) and washed with 0.01 N HCI (50 mL) and water (50 mL). The organic phase was then dried over MgS04 and the solvent was removed in vacuo. The crude product was purified by flash column chromatography with 1 : 1 EtOAc: Petrol to give 8-(fe f-butoxy)-8- oxooctanoic acid (0.41 g, 1.77 mmol, 31%) as colourless oil. 1H NMR (400 MHz, DMSO-d6) δ ppm 11.94 (br s, 1 H), 2.18 (dd, J = 14.6, 7.3 Hz, 4H), 1.54-1.42 (m, 4H), 1.40 (s, 9H), 1.34-1.20 (m, 4H)
References:
OTSUKA PHARMACEUTICAL CO., LTD.;HEIGHTMAN, Thomas, Daniel;LEBRAUD, Honorine WO2017/212329, 2017, A1 Location in patent:Page/Page column 30; 83; 84
137299-08-2
8 suppliers
inquiry

234081-94-8
60 suppliers
$35.00/250mg
3946-32-5
193 suppliers
$12.00/1g

234081-94-8
60 suppliers
$35.00/250mg

24424-99-5
869 suppliers
$13.50/25G

234081-94-8
60 suppliers
$35.00/250mg