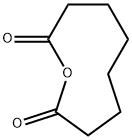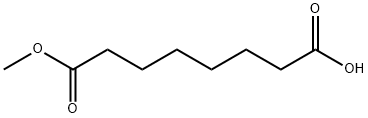
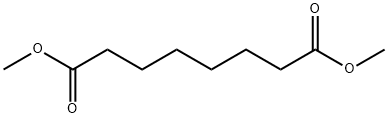
SUBERIC ACID MONOMETHYL ESTER synthesis
- Product Name:SUBERIC ACID MONOMETHYL ESTER
- CAS Number:3946-32-5
- Molecular formula:C9H16O4
- Molecular Weight:188.22
1732-09-8

3946-32-5
General method: 5.1.2 Synthesis of 7-methoxy-7-oxoheptanoic acid (84): a methanolic (150 ml) solution of KOH (5.87 g, 104.65 mmol) was slowly added dropwise to dimethyl heptanedioate (82, 16.94 g, 90 mmol) at 0 °C. The reaction mixture was stirred at room temperature for 4 hours. After completion of the reaction, the solvent was removed under reduced pressure and subsequently extracted by adding ether (100 ml) and water (200 ml). The organic phase was separated and concentrated to give yellow oil 82 (5.08 g, 30% yield). The aqueous phase was acidified to pH 3 with concentrated hydrochloric acid and extracted with ether (100 ml x 3). The combined organic layers were washed with brine (100 ml x 3), dried over magnesium sulfate and the solvent was concentrated under reduced pressure. The product was purified by silica gel column chromatography to give colorless oil 84 (5.96 g, 38% yield). Compound 85 was synthesized using the same method.5.1.2.1 Synthesis of 8-methoxy-8-oxooctanoic acid (85): 44% yield.ESI-MS m/z: 187.4 [M-H]-; 1H NMR (DMSO-d6) δ 1.21-1.32 (m, 4H), 1.43-1.53 (m, 4H), 2.18 (t, J=7.2 Hz, 2H), 2.29 (t, J=7.2Hz, 2H), 3.58 (s, 3H), 11.97 (s, 1H).
Yield:3946-32-5 80%
Reaction Conditions:
for 3 h;Reflux;
Steps:
8-Methoxy-8-oxooctanoic acid (12).
The solution of compound 9 (2.0 g, 12.8 mmol) in methanol (6.5 ml) was stirred at reflux in pressure tube during 3 h. The reaction mixture was evaporated to dryness and treated with ether. The white precipitate formed was filtered off. Yield 1.92 g(80%). 1H NMR spectrum (400 MHz, DMSO-d6), δ, ppm(J, Hz): 1.15-1.28 (4H, m, (CH2)2(CH2)2CO2CH3); 1.37-1.52 (4H, m, HO2C(CH2CH2CH2)2CO2CH3); 2.14 (2H, t,J = 7.4, CH2CO2CH3); 2.24 (2H, t, J = 7.4, CH2CO2H);3.54 (3H, s, CO2CH3, partially overlapped with water signal); 11.94 (1H, br. s, CO2H).
References:
Nikitjuka, Anna;Shestakova, Irina;Romanchikova, Nadezhda;Jirgensons, Aigars [Chemistry of Heterocyclic Compounds,2015,vol. 51,# 7,p. 647 - 657][Khim. Geterotsikl. Soedin.,2015,vol. 51,# 7,p. 647 - 657,11]

1732-09-8
191 suppliers
$8.00/5g

3946-32-5
193 suppliers
$12.00/1g

1732-09-8
191 suppliers
$8.00/5g
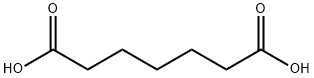
111-16-0
421 suppliers
$6.00/5g
505-48-6
447 suppliers
$8.00/10g

3946-32-5
193 suppliers
$12.00/1g

67-56-1
790 suppliers
$7.29/5ml-f

505-48-6
447 suppliers
$8.00/10g

3946-32-5
193 suppliers
$12.00/1g

1732-09-8
191 suppliers
$8.00/5g

505-48-6
447 suppliers
$8.00/10g

3946-32-5
193 suppliers
$12.00/1g