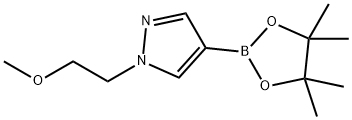
1-(2-Methoxyethyl)-4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole synthesis
- Product Name:1-(2-Methoxyethyl)-4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
- CAS Number:847818-71-7
- Molecular formula:C12H21BN2O3
- Molecular Weight:252.12
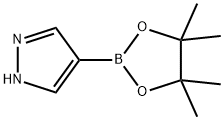
269410-08-4

6482-24-2

847818-71-7
The reaction was carried out with 4-pyrazoleboronic acid pinacol ester (1.94 g, 10 mmol) and 2-bromoethyl methyl ether (1.68 g, 12 mmol) in a microwave reactor with stirring at 160 °C for 1 hour. Potassium carbonate (2.76 g, 20 mmol) and N,N-dimethylformamide (16 mL) were added as solvents to the reaction system. After completion of the reaction, the reaction mixture was concentrated and purified by silica gel column chromatography (eluent: 30% ethyl acetate/petroleum ether) to afford 1-(2-methoxyethyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole (2.2 g, 90% yield) as a yellow oil. The product was characterized by 1H NMR (400 MHz, CDCl3): δ 1.32 (12H, s), 3.32 (3H, s), 3.75 (2H, t, J = 5.2 Hz), 4.30 (2H, t, J = 5.2 Hz), 7.77 (1H, s), 7.79 (1H, s). Mass spectral analysis: [M + H]+ m/z calculated value 253.2, measured value 253.2.

269410-08-4
394 suppliers
$5.00/1g

627-42-9
302 suppliers
$6.00/5g

847818-71-7
86 suppliers
$29.00/100mg
Yield:847818-71-7 100%
Reaction Conditions:
with caesium carbonate in N,N-dimethyl-formamide at 160; for 0.5 h;Microwave irradiation;
Steps:
B162 Compound B162a. 1-(2-Methoxyethyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazole
4-( 4,4,5,5-Tetramethyl-1 ,3,2-dioxaborolan-2-yl)-1H-pyrazole (1 00 mg, 0.52mmol), 1-chloro-2-methoxyethane (0.056 mL, 0.62 mmol) and cesium carbonate (252mg, 0.73 mmol) in DMF (1 mL) were heated in microwave reactor at 160 °C for 30 min.The reaction mixture was concentrated under reduced pressure and purified by silica gelchromatography (ISCO, hexanes/ethyl acetate 0-100% over 15 min) to isolate CompoundB162a (130 mg, 100% yield). HPLC: RT = 1.0 min (LCMS Method M). MS (ES): m/z= 253.0 [M+H( 1H NMR (500MHz, CHLOROFORM-d) 8 8.04 (s, 1H), 7.83 (d,J=18.4 Hz, 1H), 4.37 (t, J=5.2 Hz, 1H), 3.78 (t, J=5.2 Hz, 1H), 3.38-3.31 (m, 2H), 2.98(s, 3H), 2.90 (s, 3H), 1.34 (s, 6H), 1.26 (s, 3H).
References:
WO2014/15088,2014,A1 Location in patent:Paragraph 00223

269410-08-4
394 suppliers
$5.00/1g

6482-24-2
297 suppliers
$9.00/1g

847818-71-7
86 suppliers
$29.00/100mg

73183-34-3
587 suppliers
$6.00/5g
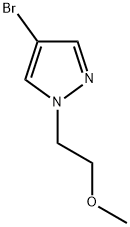
847818-49-9
44 suppliers
inquiry

847818-71-7
86 suppliers
$29.00/100mg
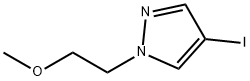
1407429-97-3
8 suppliers
inquiry

73183-34-3
587 suppliers
$6.00/5g

847818-71-7
86 suppliers
$29.00/100mg

269410-08-4
394 suppliers
$5.00/1g

109-86-4
574 suppliers
$11.00/25g

847818-71-7
86 suppliers
$29.00/100mg