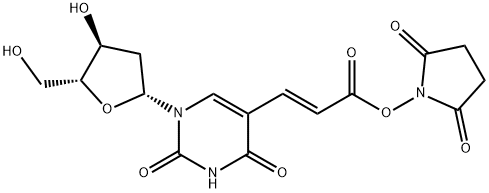
(E)-5-[2-(2-CARBOXYVINYL)]-2'-DEOXYURIDINE N-HYDROXY-SUCCIMIDE ESTER synthesis
- Product Name:(E)-5-[2-(2-CARBOXYVINYL)]-2'-DEOXYURIDINE N-HYDROXY-SUCCIMIDE ESTER
- CAS Number:869355-24-8
- Molecular formula:C16H17N3O9
- Molecular Weight:395.32
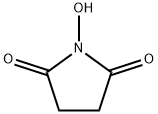
6066-82-6
805 suppliers
$5.00/10g
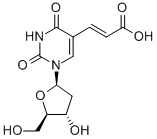
74131-06-9
45 suppliers
$70.00/100mg
![(E)-5-[2-(2-CARBOXYVINYL)]-2'-DEOXYURIDINE N-HYDROXY-SUCCIMIDE ESTER](/CAS/GIF/869355-24-8.gif)
869355-24-8
14 suppliers
inquiry
Yield:-
Reaction Conditions:
with 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in N,N-dimethyl-formamide at 25; for 3 h;Product distribution / selectivity;
Steps:
1
Next, 1.20 g of (E)-5-(2-carboxy vinyl)-2'-deoxyuridine 101 (with a molecular weight of 298.25), 925 mg of N-hydroxysuccinimide (with a molecular weight of 115.09), and 1.54 g of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (with a molecular weight of 191.70) were placed in a recovery flask containing a stirring bar, and 20 mL of DMF was added thereto, which then was stirred at 25°C for 16 hours. About 1 mL of acetic acid was added thereto and 300 mL of methylene chloride and 100 mL of ultrapure water were added thereto, which was then stirred vigorously. The aqueous layer was removed and further 100 mL of ultrapure water was added, which then was washed twice in the same manner. The precipitate thus produced was filtered, washed with methylene chloride, and then dried under reduced pressure. The solvent was evaporated from the filtrate, methylene chloride was added to the precipitate thus produced, and the precipitate was then recovered in the same manner as described above. The precipitates thus recovered were collected and then suspended in 80 mL of acetonitrile. This was stirred vigorously. Then 3.0 mL of tris(2-aminoethyl)amine (with a molecular weight of 146.23, d=0.976) was added all at once, which further was stirred at 25°C for 10 minutes. Thereafter, 4.8 mL of ethyl trifluoroacetate (with a molecular weight of 142.08, d=1.194) was added and further 5.6 mL of triethylamine (with a molecular weight of 101.19, d=0.726) was added thereto, which was stirred at 25°C for three hours. The solvent was evaporated and the product thus obtained was purified with a silica gel column (5-10% MeOH/CH2Cl2). The solvent was evaporated, the product thus obtained was dissolved in a small amount of acetone, and ether then was added thereto. As a result, white precipitate was produced. This was filtered and then washed with ether. Thereafter, this was dried under reduced pressure. Thus 884 mg (33.5%) of target substance (Compound 102) was obtained. The same synthesis as described above was carried out except for slight changes in the amounts of, for example, raw materials and solvents to be used, the reaction time, and the steps to be taken. As a result, the yield was improved up to 37%. That is, 597 mg (2.0 mmol) of (E)-5-(2-carboxy vinyl)-2'-deoxyuridine 101 (with a molecular weight of 298.25), 460 mg (4.0 mmol) of N-hydroxysuccinimide (with a molecular weight of 115.09), and 767 mg (4.0 mmol) of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (with a molecular weight of 191.70) were placed in a recovery flask containing a stirring bar. Thereafter, 5.0 mL of DMF was added thereto, which was stirred at 25°C for three hours. About 0.5 mL of acetic acid was added and 100 mL of methylene chloride and 100 mL of ultrapure water were added thereto, which was stirred vigorously. The precipitate thus produced was filtered, washed with water, and then dried under reduced pressure throughout the night. The resultant white residue was suspended in 50 mL of acetonitrile, which was stirred vigorously. Then, 3.0 mL (20 mmol) of tris(2-aminoethyl)amine (with a molecular weight of 146.23, d=0.976) was added thereto all at once, which further was stirred at 25°C for 10 minutes. Thereafter, 4.8 mL of ethyl trifluoroacetate (with a molecular weight of 142.08, d=1.194) was added and further 5.6 mL (40 mmol) of triethylamine (with a molecular weight of 101.19, d=0.726) was added thereto, which was then stirred at 25°C for 16 hours. The solvent was evaporated and the product thus obtained was purified with a silica gel column (5-10% MeOH/CH2Cl2). The solvent was evaporated, the product thus obtained was dissolved in a small amount of acetone, and ether was then added thereto. As a result, white precipitate was produced. This was filtered and then washed with ether. Thereafter, this was dried under reduced pressure. Thus 453 mg (37%) of target substance (Compound 102) was obtained as white powder. The instrumental analytical values of Compound 102 are indicated below. Further, a 1HNMR spectrum diagram is shown in FIG. 2. 2-[2-[N,N-bis(2-trifluoroacetamidoethyl)]-aminoethyl]carbamoyl-(E) -vinyl)-2'-deoxyuridine (Compound 102): 1HNMR(CD3OD): δ8.35 (s,1H), 7.22 (d, J=15.6Hz, 1H), 7.04 (d, J=15.6Hz, 1H), 6.26 (t, J=6.6Hz, 1H), 4.44-4.41 (m, 1H), 3.96-3.94 (m, 1H), 3.84 (dd, J=12.2, 2.9Hz, 1H), 3.76 (dd, J=12.2, 3.4Hz, 1H), 3.37-3.30 (m, 6H), 2.72-2.66 (m, 6H), 2.38-2.23 (m, 2H).13CNMR(CD3OD): δ169.3, 163.7, 159.1 (q,J=36.4Hz), 151.2, 143.8, 134.3, 122.0, 117.5 (q,J=286Hz), 110.9, 89.1, 87.0, 71.9, 62.5, 54.4, 53.9, 41.7, 38.9, 38.7. HRMS (ESI) calcd for C22H29F6N6O8 ([M+H]+) 619.1951, found 619.1943.
References:
EP2130835,2009,A1 Location in patent:Page/Page column 40-41
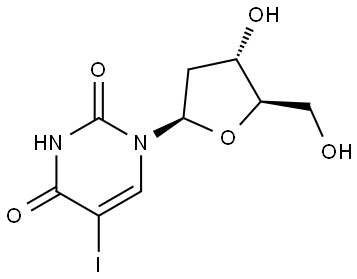
54-42-2
466 suppliers
$6.00/250mg
![(E)-5-[2-(2-CARBOXYVINYL)]-2'-DEOXYURIDINE N-HYDROXY-SUCCIMIDE ESTER](/CAS/GIF/869355-24-8.gif)
869355-24-8
14 suppliers
inquiry
![2-Propenoic acid, 3-[1-(2-deoxy-β-D-erythro-pentofuranosyl)-1,2,3,4-tetrahydro-2,4-dioxo-5-pyrimidinyl]-, methyl ester](/CAS/20210305/GIF/96244-97-2.gif)
96244-97-2
2 suppliers
inquiry
![(E)-5-[2-(2-CARBOXYVINYL)]-2'-DEOXYURIDINE N-HYDROXY-SUCCIMIDE ESTER](/CAS/GIF/869355-24-8.gif)
869355-24-8
14 suppliers
inquiry