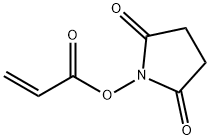
N-ACRYLOXYSUCCINIMIDE synthesis
- Product Name:N-ACRYLOXYSUCCINIMIDE
- CAS Number:38862-24-7
- Molecular formula:C7H7NO4
- Molecular Weight:169.13
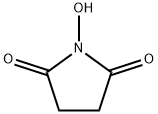
6066-82-6
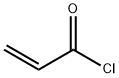
814-68-6

38862-24-7
N-hydroxysuccinimide (10 g, 0.086 mol) and triethylamine (13.2 g, 0.129 mol) were dissolved in chloroform (130 mL) at 0 °C. Stirring was maintained and acryloyl chloride (8.6 g, 0.094 mol) was slowly added dropwise to the reaction mixture, the dropwise process was controlled to be completed within 2 hours. After the reaction was completed, stirring was continued at 0 °C for 30 min. Subsequently, the reaction solution was washed twice with 60 mL of saturated aqueous NaCl, and the organic phase was dried with MgSO4, filtered and concentrated to a residual volume of about 30 mL. to the concentrate was added a solvent mixture of ethyl acetate/pentane (14 mL, 1:3, v/v/v), and the mixture was kept at 0 °C to promote the crystallization of N-acryloyloxysuccinimide overnight, resulting in the target 70% yield of the Product. The structure of the product was confirmed by 1H NMR (CDCl3) with chemical shifts of 2.95 (s, 4H, CH2CH2), 6.20 (m, 1H, CH=CH2), 6.4 (m, 1H, CH=CH2) and 6.75 (m, 1H, CH=CH2), respectively.

6066-82-6
806 suppliers
$5.00/10g
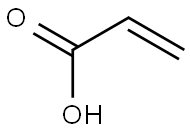
79-10-7
737 suppliers
$20.16/100 mL
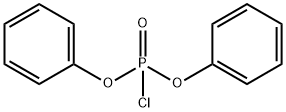
2524-64-3
393 suppliers
$14.00/5g

38862-24-7
166 suppliers
$8.00/250mg
Yield:-
Reaction Conditions:
in dichloromethane;di-isopropyl ether;water;acetone
Steps:
11 EXAMPLE 11
EXAMPLE 11 0.72 g (0.010 mol) of acrylic acid was initially introduced into 30 ml of acetone and 1.27 g (0.011 mol) of solid N-hydroxysuccinimide were added. The resulting solution was treated with 3.36 g (0.040 mol) of solid sodium hydrogencarbonate and the white suspension thus obtained was stirred at room temperature. A solution of 3.22 g (0.012 mol) of diphenyl chlorophosphate in 10 ml of acetone was then added dropwise and the reaction mixture was stirred at room temperature for 24 hours and at 50° C. for a further 3 hours to complete the reaction, the thickening suspension being diluted with 25 ml of acetone. The diluent was then removed in vacuo and the residue was taken up in 60 ml of dichloromethane. Undissolved solid was filtered and washed twice with 10 ml each of dichloromethane. The combined dichloromethane phases were washed with 40 ml of water and, after phase separation, evaporated in vacuo. 1.53 g of solid crude product were obtained, which was washed by stirring with 15 ml of diisopropyl ether for 3 hours. The white solid which remained was filtered off, washed twice with 3 ml of diisopropyl ether each time and dried in vacuo. 1.1 g (65%) of acrylic acid succinimidyl ester were obtained. M.p.: 63°-68° C.
References:
DSM Chemie Linz GmbH US5734064, 1998, A

6066-82-6
806 suppliers
$5.00/10g

814-68-6
396 suppliers
$21.21/5gm:

38862-24-7
166 suppliers
$8.00/250mg

6066-82-6
806 suppliers
$5.00/10g

79-10-7
737 suppliers
$20.16/100 mL

38862-24-7
166 suppliers
$8.00/250mg
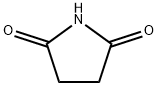
123-56-8
529 suppliers
$6.00/25g

814-68-6
396 suppliers
$21.21/5gm:

38862-24-7
166 suppliers
$8.00/250mg