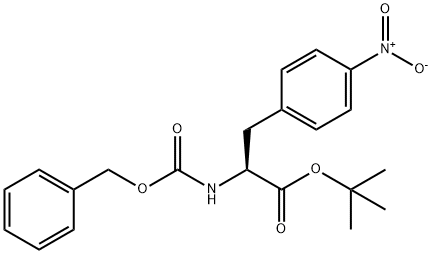
(S)-tert-Butyl 3-(4-aminophenyl)-2-(((benzyloxy)carbonyl)amino)propanoate synthesis
- Product Name:(S)-tert-Butyl 3-(4-aminophenyl)-2-(((benzyloxy)carbonyl)amino)propanoate
- CAS Number:869882-72-4
- Molecular formula:C21H26N2O4
- Molecular Weight:370.4421
869882-71-3
8 suppliers
inquiry

869882-72-4
9 suppliers
$505.24/5MG
Yield:869882-72-4 84%
Reaction Conditions:
with hydrogen;platinum(IV) oxide in ethanol at 20; under 2585.81 - 3102.97 Torr; for 3 h;
Steps:
1
Protected 4-nitrophenylalanine (73 g, 0.18 m) is dissolved in ethanol (500 ml) and platinum oxide catalyst (1.5 g) is added. The resulting solution is vigorously stirred in hydrogen atmosphere (50-60 psi) at ambient temperature until further hydrogen adsorption ceased (3 hr). The catalyst is filtered off and the filtrate is evaporated to dryness, the residue is taken up in ethyl acetate (200 ml) and filtered through plug of silica gel (2×2 in) using ethyl acetate-hexane mixture (3:2, 2L) to rinse silica. The filtrate is concentrated to approx. 200 ml and hexane (500 ml) is added. The crystalline product is filtered off, rinsed with cold solvent and air-dried. Yield-56 g, 84%.
References:
US2006/13799,2006,A1 Location in patent:Page/Page column 53
![L-Alanine, 3-iodo-N-[(phenylmethoxy)carbonyl]-, 1,1-dimethylethyl ester](/CAS/20200611/GIF/190579-73-8.gif)