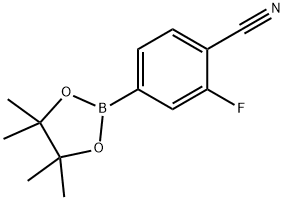
2-Fluoro-4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)benzonitrile synthesis
- Product Name:2-Fluoro-4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)benzonitrile
- CAS Number:870238-67-8
- Molecular formula:C13H15BFNO2
- Molecular Weight:247.07
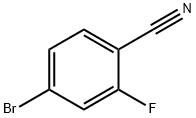
105942-08-3

73183-34-3

870238-67-8
4-Bromo-2-fluorobenzonitrile (4.0 g, 20 mmol), pinacol ester of bisboronic acid (3.8 g, 30 mmol), and potassium acetate (6.1 g, 60 mmol) were suspended in dimethyl sulfoxide (DMSO, 50 mL) under nitrogen protection. Subsequently, [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (Pd(dppf)Cl2, 1.5 g, 0.2 mmol) was added. The reaction mixture was stirred at 80 °C for 4 hours. After completion of the reaction, the mixture was diluted with water (100 mL) and extracted with ethyl acetate (100 mL × 3). The organic layers were combined and washed sequentially with water (50 mL × 3) and saturated saline (50 mL), then dried with anhydrous sodium sulfate. After drying, the filtrate was filtered and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent ratio: petroleum ether/ethyl acetate = 50:1 to 10:1) to afford the target product 4-cyano-3-fluorophenylboronic acid pinacol ester (56-a, 3.6 g, 73% yield). The product was characterized by 1H-NMR (400 MHz, CD3OD): δ 7.62 (m, 3H), 1.35 (s, 12H) ppm.

105942-08-3
382 suppliers
$7.00/5g

73183-34-3
587 suppliers
$6.00/5g

870238-67-8
98 suppliers
$11.00/250mg
Yield:870238-67-8 89%
Reaction Conditions:
with palladium (II) [1,1'-bis(diphenylphosphanyl)ferrocene] dichloride;anhydrous potassium acetate in 1,4-dioxane;dimethyl sulfoxide at 105; for 3 h;Inert atmosphere;
Steps:
xiv.a 2-Fluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzonitrile I37
A mixture of 4-bromo-2-fluorobenzonitrile (1.0 g, 5.0 mmol), 4,4,4',4',5,5,5',5'-octamethyl-2,2'-bi(1,3,2-dioxaborolane) (1.3 g, 5.0 mmol), potassium acetate (5.9 g, 20.0 mmol) and Pd(dppf)Cl2 (2.0 g, 1.0 mmol) in DMSO (50 ml.) and 1,4-dioxane (10 ml.) was heated at 105 °C under N2 for 3 h. [82] The mixture was diluted with EtOAc (200 ml.) and washed with water (100 ml. x 3). The organic layer was dried over anhydrous Na2SO4, filtered, concentrated under reduced pressure and the residue was purified by column chromatography (Pet. ether/EtOAc= 100/0 to 50/1) to give the title compound (1.1 g, 89%) as a white solid, which was used directly in the next step.
References:
WO2019/243491,2019,A1 Location in patent:Page/Page column 81-82
843663-18-3
228 suppliers
$8.00/250mg
76-09-5
397 suppliers
$10.00/1g

870238-67-8
98 suppliers
$11.00/250mg
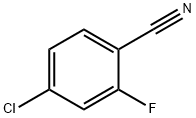
57381-51-8
191 suppliers
$6.00/1g
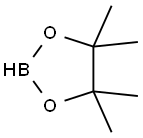
25015-63-8
216 suppliers
$22.39/5G

870238-67-8
98 suppliers
$11.00/250mg
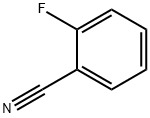
394-47-8
479 suppliers
$5.00/5g