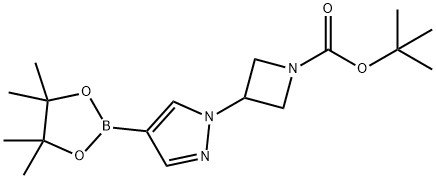
TERT-BUTYL 3-(4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-1H-PYRAZOL-1-YL)AZETIDINE-1-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 3-(4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-1H-PYRAZOL-1-YL)AZETIDINE-1-CARBOXYLATE
- CAS Number:877399-35-4
- Molecular formula:C17H28BN3O4
- Molecular Weight:349.23
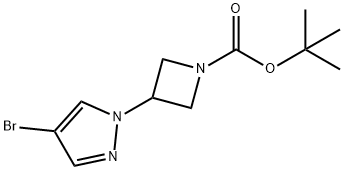
877399-34-3

73183-34-3

877399-35-4
Synthesis of tert-butyl 3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)azetidine-1-carboxylate (2-8): tert-butyl 3-(4-bromo-1H-pyrazol-1-yl)azetidine-1-carboxylate (2-8) was synthesized by dissolving compound 3-(4-bromo-1H-pyrazol-1-yl)tert-butyl azetidine-1-carboxylate (2-8) (225 mg, 0.74 mmol), biboronic acid pinacol ester (2-7) (227 mg, 0.89 mmol) and potassium acetate (247 mg, 2.52 mmol) were dissolved in 3 mL of dimethyl sulfoxide (DMSO) and the reaction mixture was purged with nitrogen for 15 min. Subsequently, dichlorobis(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) (PdCl2(dppf)2-CH2Cl2) (30 mg, 2.52 mmol) was added. The resulting mixture was stirred at 80 °C overnight under nitrogen protection. After completion of the reaction, it was cooled to room temperature and the mixture was filtered through a diatomaceous earth pad and washed well with ethyl acetate. The filtrate was washed sequentially with water (2 x 50 mL) and brine (50 mL). The organic layer was dried with anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by silica gel column chromatography with the eluent being petroleum ether: ethyl acetate (3:2). The filtrate was concentrated under reduced pressure to give 250 mg of the target product 2-8 as a clear oil (97% yield).1H NMR (400 MHz, chloroform-d) δ ppm 1.18-1.27 (m, 9H), 1.28-1.34 (m, 6H), 1.41-1.49 (m, 6H), 4.22-4.33 (m, 2H), 4.36 (t, J = 8.59 Hz, 2H), 4.98-5.13 (m, 1H), 7.83 (s, 2H).

877399-34-3
64 suppliers
$54.00/100mg

73183-34-3
587 suppliers
$6.00/5g

877399-35-4
76 suppliers
$85.00/100mg
Yield: 97%
Reaction Conditions:
with potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 in dimethyl sulfoxide at 80;
Steps:
59
tert-Butyl 3-[4-(4,4,5,5-tetramethyl-1,3-dioxoborolan-2-yl)-1H-pyrazol-1-yl]azetidine-1-carboxylate (2-8): A reaction mixture of compound (2-6) (225 mg, 0.74 mmol) and bis(pinacolate)diboron (2-7, 227 mg, 0.89 mmol) with KOAc (247 mg, 2.52 mmol) in 3 mL of DMSO was purged with N2 for 15 minutes, then PdCl2(dppf)2CH2Cl2 (30 mg, 2.52 mmol) was added. The resulting mixture was stirred at 80° C. under N2 for overnight. After it cooled down to room temperature, the mixture was filtered through Celite pad and washed well with EtOAc. The filtrate was extracted with H2O (2*50 mL), brine (50 mL). The organic layer was dried (Na2SO4), then concentrated by vacuum. The residue was then filtered through silica gel pad, eluted with hexane:EtOAc/3:2. The filtrate was concentrated by vacuum to give 250 mg of 2-8) as a clear oil (97% yield). 1H NMR (400 MHz, chloroform-D) δ ppm 1.18-1.27 (m, 9H) 1.28-1.34 (m, 6H) 1.41-1.49 (m, 6H) 4.22-4.33 (m, 2H) 4.36 (t, J=8.59 Hz, 2H) 4.98-5.13 (m, 1H) 7.83 (s, 2H).
References:
AGOURON PHARMACEUTICALS, INC. US2006/46991, 2006, A1 Location in patent:Page/Page column 57-58
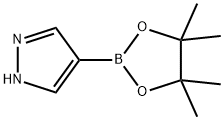
269410-08-4
395 suppliers
$5.00/1g
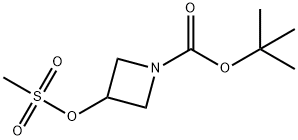
141699-58-3
113 suppliers
$6.00/250mg

877399-35-4
76 suppliers
$85.00/100mg

269410-08-4
395 suppliers
$5.00/1g
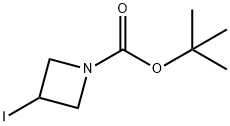
254454-54-1
262 suppliers
$15.00/1g

877399-35-4
76 suppliers
$85.00/100mg
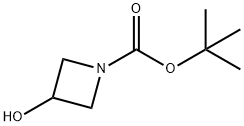
141699-55-0
390 suppliers
$6.00/1g

877399-35-4
76 suppliers
$85.00/100mg

141699-58-3
113 suppliers
$6.00/250mg

877399-35-4
76 suppliers
$85.00/100mg