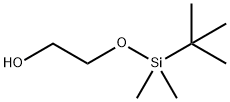
(TERT.-BUTYLDIMETHYLSILYLOXY)ETHANOL synthesis
- Product Name:(TERT.-BUTYLDIMETHYLSILYLOXY)ETHANOL
- CAS Number:102229-10-7
- Molecular formula:C8H20O2Si
- Molecular Weight:176.33
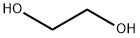
107-21-1

18162-48-6

102229-10-7
A solution of anhydrous THF (100 mL) with ethylene glycol (20.0 g, 0.322 mol) was added slowly and dropwise to an anhydrous THF (500 mL) suspension of NaH (12.9 g, 0.322 mol, 60% dispersed in mineral oil). After stirring the reaction mixture for 1 h at room temperature, tert-butyldimethylchlorosilane (TBSCl, 48.59 g, 0.322 mol) was added and stirring was continued for 1 h at room temperature. Upon completion of the reaction, the reaction was quenched with 10% aqueous K2CO3 (100 mL) and subsequently extracted with methyl tert-butyl ether (MTBE, 3 x 300 mL). The organic phases were combined, dried with anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography with gradient elution using a petroleum ether solution of 1% to 33% ethyl acetate to afford 2-((tert-butyldimethylmethylsilyl)oxy)ethanol (55.0 g, 96% yield) as a colorless oil.

18162-48-6
678 suppliers
$9.00/5g

102229-10-7
179 suppliers
$10.00/1g
Yield:102229-10-7 97%
Reaction Conditions:
Stage #1:ethylene glycol with sodium hydride in tetrahydrofuran at 20; for 1 h;
Stage #2:tert-butyldimethylsilyl chloride in tetrahydrofuran at 20; for 2 h;
Steps:
108
To a solution of 2 g (32.22 mmol) of ethane-1 ,2-diol in 50 ml of dried tetrahydrofuran, 1.29 g (32.25 mmol) of sodium hydride (60% in mineral oil) were added in several portions. The mixture was stirred for 1 hour at room temperature. After this time a solution of tert- butylchlorodimethylsilane (4.9 g, 32.51 mmol) in 15 ml of dried tetrahydrofuran was slowly added. The reaction mixture was stirred at room temperature for 2 hours. After this time, the reaction mixture was diluted with 120 ml of diethyl ether and treated with 120 ml of NaHCO3 (4% aqueous solution). The organic phase was separated, washed with NaHCO3 (4% aqueous solution), water and brine, dried (MgSO4), and concentrated to dryness. The title product was obtained as an oil (5.51 , 97%).
References:
LABORATORIOS, ALMIRALL S.A. WO2007/124898, 2007, A1 Location in patent:Page/Page column 78-79
107-21-1
1381 suppliers
$10.00/25g

18162-48-6
678 suppliers
$9.00/5g

102229-10-7
179 suppliers
$10.00/1g
107-21-1
1381 suppliers
$10.00/25g

18162-48-6
678 suppliers
$9.00/5g
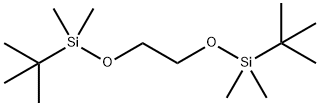
66548-22-9
46 suppliers
inquiry

102229-10-7
179 suppliers
$10.00/1g
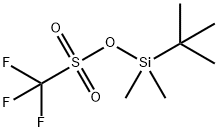
69739-34-0
369 suppliers
$10.00/1g
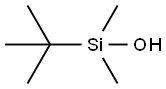
18173-64-3
131 suppliers
$15.00/1g

102229-10-7
179 suppliers
$10.00/1g