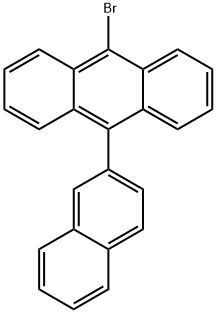
9-Bromo-10-(2-naphthyl)anthracene synthesis
- Product Name:9-Bromo-10-(2-naphthyl)anthracene
- CAS Number:474688-73-8
- Molecular formula:C24H15Br
- Molecular Weight:383.28
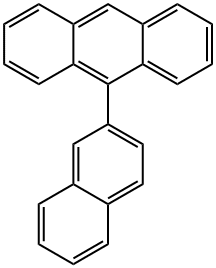
7424-72-8

474688-73-8
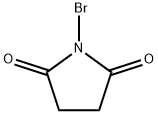
The general procedure for the synthesis of 9-bromo-10-(2-naphthalenyl)anthracene from 9-(naphthalen-2-yl)anthracene was as follows: 9-(2-naphthalenyl)anthracene (2.7 g, 8.9 mmol) was suspended in anhydrous N,N-dimethylformamide (DMF, 50 mL). To this suspension was slowly added anhydrous DMF solution (6 mL) dissolved in N-bromosuccinimide (NBS, 1.7 g, 9.6 mmol, 1.1 equiv). The resulting mixture was stirred and reacted for 10 h at room temperature and then left to stand overnight. Upon completion of the reaction, the reaction mixture was diluted with deionized water (50 mL). The resulting yellowish solid product was separated by filtration and washed with methanol to give the final target compound 9-bromo-10-(2-naphthyl)anthracene (3.2 g, 94% yield). The structure of the product was confirmed by 1H-NMR (CDCl3, TMS) with chemical shifts δ: 7.2-7.7 (9H, m), 7.8-8.1 (4H, m), 8.62 (2H, d, J = 8 Hz).
128-08-5
893 suppliers
$5.00/5G

7424-72-8
58 suppliers
$75.00/100mg

474688-73-8
232 suppliers
$15.00/1g
Yield:474688-73-8 99%
Reaction Conditions:
in water;N,N-dimethyl-formamide;
Steps:
S.5 Synthesis Example 5
Synthesis Example 5 (Synthesis of 9-bromo-10-(naphthalene-2-yl)anthracene) 23.1 g of 9-(naphthalene-2-yl)anthracene was dispersed into 250 ml of DMF (dimethylformamide), and then 14.9 g of NBS (N-bromosuccinimide) in DMF solution (150 ml) was dropped therein. After stirred at room temperature for 7 hours, it was left over a night. 200 ml of water was added thereto, and then the crystal precipitated was separated by filtration. Subsequently it was washed by ethanol adequately, followed by drying, and 28.8 g of 9-bromo-10-(naphthalene-2-yl)anthracene of beige color crystal was obtained (Yield: 99%).
References:
US2007/152565,2007,A1
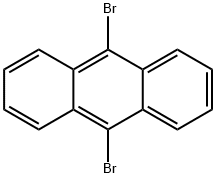
523-27-3
392 suppliers
$6.00/5g
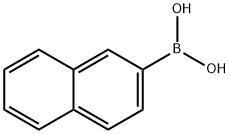
32316-92-0
476 suppliers
$6.00/1g

474688-73-8
232 suppliers
$15.00/1g

7424-72-8
58 suppliers
$75.00/100mg

474688-73-8
232 suppliers
$15.00/1g
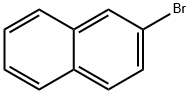
580-13-2
506 suppliers
$6.00/1g

474688-73-8
232 suppliers
$15.00/1g

32316-92-0
476 suppliers
$6.00/1g

474688-73-8
232 suppliers
$15.00/1g