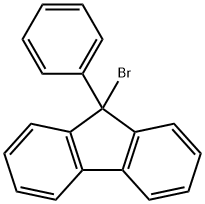
9-Bromo-9-phenylfluorene synthesis
- Product Name:9-Bromo-9-phenylfluorene
- CAS Number:55135-66-5
- Molecular formula:C19H13Br
- Molecular Weight:321.21
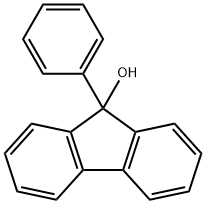
25603-67-2

55135-66-5
(1) Prepare a 10L reactor, first add 6L of toluene, turn on the stirring; under the condition of 20-25℃, slowly add 9-phenyl-9-fluorenol (1kg, 3.87mol), stirring until completely dissolved, forming a milky white solution; (2) Slowly add 48% hydrobromic acid (2.9L, 25.5mol) to the reaction system, and the reaction solution became light yellow and showed a turbid state; (3) Place the reaction system in a water bath, heating to 50-60℃, and continue to react for 48 hours to observe the gradual deepening of the color of the reaction solution; (4) Monitor the reaction process by gas chromatography (GC). (3) The reaction system was placed in a water bath, heated to 50-60 ℃, and the reaction continued for 48 hours, observing the gradual deepening of the color of the reaction solution; (4) The reaction process was monitored by gas chromatography (GC), and the reaction was terminated when the content of the main raw material 9-phenyl-9-fluorenyl alcohol decreased to 3%; (5) The water bath was removed, and the reaction solution was cooled down to room temperature, and was then transferred to the 20L reactor for post-processing: stirring, standing, and layering; the lower part of the water bath was removed, and the reaction solution was cooled to room temperature. After standing, stratification; the lower aqueous phase was extracted once with 2L of toluene, the organic phases were combined, washed sequentially with 4L of saturated sodium carbonate solution, 4L of water, 4L of saturated saline, and finally the organic phase was dried with 100g of anhydrous magnesium sulfate; (6) after drying for 4 hours, filtration was carried out, and the filter cake was discarded. The filtrate was concentrated to dryness under reduced pressure at 45°C to obtain 1.1kg of white solid with 96% purity by GC; (7) Recrystallization and purification of 9-bromo-9-phenylfluorene: 1.1kg of crude product was added into a 10L reactor, 7L of hexane was added, stirred and heated to reflux, and after the solution became transparent, 50g of activated charcoal was added to decolorize the solution, stirred for 30 minutes, and the filtrate was heat-filtered at 65°C, and the filtrate was naturally The filtrate was cooled to room temperature, then placed in an ice-salt bath and cooled to -5~-10 ℃, and left to stand for 4 hours. Filtering to obtain white crystals, wet weight 1.25 kg. 50 ℃ vacuum drying for 12 hours, to obtain 1.02 kg of yellow crystalline powder, GC purity of 99%, melting point of 99 to 100 ℃, the total yield of 82%.
108-86-1
522 suppliers
$10.00/5g
486-25-9
488 suppliers
$5.00/25g

55135-66-5
191 suppliers
$7.00/250mg
Yield:-
Steps:
Multi-step reaction with 2 steps
1.1: n-butyllithium / diethyl ether; hexane / 0.83 h / 0 °C / Inert atmosphere
1.2: 2.42 h / 0 - 24 °C / Inert atmosphere
2.1: hydrogen bromide / toluene; water / 24 h / 20 - 25 °C / Inert atmosphere
References:
Jamison;Lubell;Dener;Krisché;Rapoport [Organic Syntheses,1993,vol. 71,p. 220 - 220]

25603-67-2
170 suppliers
$7.00/250mg

55135-66-5
191 suppliers
$7.00/250mg
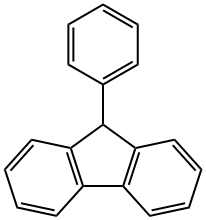
789-24-2
30 suppliers
$144.00/25mg

55135-66-5
191 suppliers
$7.00/250mg
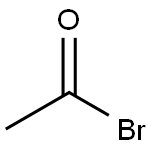
506-96-7
322 suppliers
$22.39/25G

25603-67-2
170 suppliers
$7.00/250mg

55135-66-5
191 suppliers
$7.00/250mg

486-25-9
488 suppliers
$5.00/25g

55135-66-5
191 suppliers
$7.00/250mg