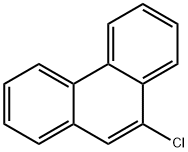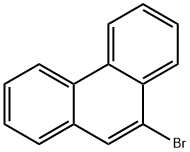
9-Bromophenanthrene synthesis
- Product Name:9-Bromophenanthrene
- CAS Number:573-17-1
- Molecular formula:C14H9Br
- Molecular Weight:257.13
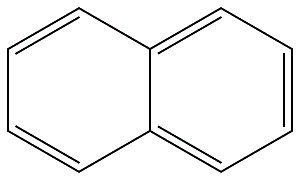
91-20-3
526 suppliers
$14.00/25g

573-17-1
317 suppliers
$6.00/5g
Yield:-
Steps:
Multi-step reaction with 4 steps
1: N-Bromosuccinimide; gold(III) chloride / 1,2-dichloro-ethane / 15 h / 80 °C / Inert atmosphere
2: sodium amide; potassium tert-butylate / tetrahydrofuran / 15 h / 50 °C / Inert atmosphere
3: perrhenic acid anhydride; triphenyl phosphite / toluene / 80 °C / Inert atmosphere
4: N-Bromosuccinimide; gold(III) chloride / 1,2-dichloro-ethane / 15 h / 80 °C / Inert atmosphere
References:
Murai, Masahito;Ogita, Takuya;Takai, Kazuhiko [Chemical Communications,2019,vol. 55,# 16,p. 2332 - 2335]
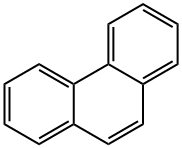
85-01-8
300 suppliers
$15.00/10g

573-17-1
317 suppliers
$6.00/5g
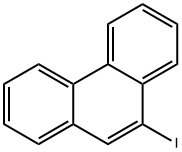
17024-12-3
72 suppliers
$52.00/1g

573-17-1
317 suppliers
$6.00/5g
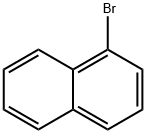
90-11-9
539 suppliers
$5.00/10g

573-17-1
317 suppliers
$6.00/5g