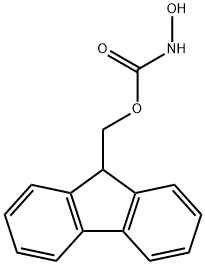
9-FLUORENYLMETHYL N-HYDROXYCARBAMATE synthesis
- Product Name:9-FLUORENYLMETHYL N-HYDROXYCARBAMATE
- CAS Number:190656-01-0
- Molecular formula:C15H13NO3
- Molecular Weight:255.27
82911-69-1

190656-01-0
The general procedure for the synthesis of (9H-fluoren-9-yl)methyl hydroxycarbamate from 9-fluorenylmethyl-N-succinimidyl carbonate was as follows: first, hydroxylamine hydrochloride (834 mg, 12 mmol) was dissolved in 40 mL of aqueous sodium bicarbonate (2.2 g, 26 mmol), and the solution was cooled to 5 °C. Subsequently, N-(9-fluorenylmethoxycarbonyloxy)succinimide (Fmoc-OSu, 4.0 g, 12 mmol) dissolved in 40 mL of ethyl acetate was slowly added dropwise to the rapidly stirring hydroxylamine solution under ice bath conditions. The reaction mixture was stirred at room temperature for 4 h. The progress of the reaction was monitored by thin-layer chromatography (TLC) (unfolding reagent: ethyl acetate/hexane = 1:1, Rf = 0.4). After completion of the reaction, the aqueous layer was separated and the organic layer was washed sequentially with saturated aqueous potassium bisulfate and brine. The organic extract was concentrated under high vacuum and subsequently ground in hexane to afford N-Fmoc-protected hydroxylamine (Fmoc-NHOH) as a white crystalline solid in 80% yield. The structure of the product was confirmed by 1H NMR (JNM-LA300 spectrometer, JEOL Ltd, Tokyo, Japan): δH (CDCl3) 4.21 (1H, t, Fmoc CH), 4.32 (2H, d, Fmoc CH2), 7.28-7.43, 7.68, 7.86 (8H, m, Fmoc Ar.CH). 8.77 (1H, s, NH), 9.75 (1H, br s, OH). Next, the coupling reaction of Fmoc-NHOH (2 eq.) with 2-chlorotrityl chloride (CTC) resin (1.43 mmol/g) in dichloromethane (DCM) was carried out for 48 h with the addition of N,N'-diisopropylethylamine (DIPEA; 4 eq.) as a base. The CTC resin containing Fmoc-NHOH was treated with 10% DIPEA/methanol (v/v) to seal the remaining chloride groups. After filtration, the loading of the resin was determined by Fmoc titration to be 1.0 mmol/g. Subsequently, the resin was treated with 20% piperidine/N-methyl-2-pyrrolidone (NMP) for 30 min to remove the Fmoc protecting groups, and then Fmoc-1-Pro-OH or Fmoc-1-Phe-OH (2 equiv.) was added to the resin at 2-(1H-7-azabenzotriazole-1- based)-1,1,3,3-tetramethyluronium hexafluorophosphate (HATU), 1-hydroxy-7-azabenzotriazole (HOAt), and DIPEA (4 eq.) in the presence of 2-(1H-7-azabenzotriazole-1-yl) for 1.5 h at room temperature. After removing the Fmoc protecting group again with 20% piperidine/NMP, HCA (2 eq.) was coupled with the amino acid-anchored resin in the presence of benzotriazol-1-yl-oxo-tris-(dimethylamino)-hexafluorophosphate (BOP; 2 eq.), hydroxybenzotriazole (HOBt; 2 eq.), and DIPEA (3 eq.) for 5 hr. The final product was cleaved from the resin by 30% trifluoroacetic acid (TFA)/DCM (v/v) for 1 hour. The resin was filtered and the filtrate was concentrated under high vacuum and then precipitated with cold ether. The resulting HCA-Phe-NHOH and HCA-Pro-NHOH were analyzed by QUATTRO Triple Quardrupole Tandem mass spectrometer (Micromass & Waters, Milford, MA, USA) at the National Instrumentation Center for Environmental Management (NICEM) for structural confirmation: CA-Phe-NHOH (m/z calculated: 343.1 [M+H]+; measured: 343.0), CA-Pro-NHOH (m/z calculated: 293.1 [M+H]+; measured: 293.1), DHCA-Phe- NHOH (m/z calculated value: 345.1 [M+H]+; measured value: 345.1), DHCA-Pro-NHOH (m/z calculated value: 295.1 [M+H]+; measured value: 295.1), pCoA-Phe-NHOH (m/z calculated value: 327.1 [M+H]+; measured value: 327.1), pCoA- Pro-NHOH (m/z calculated value: 277.1 [M+H]+; measured value: 277.0), FA-Phe-NHOH (m/z calculated value: 357.1 [M+H]+; measured value: 357.1), FA-Pro-NHOH (m/z calculated value: 307.1 [M+H]+; measured value: 307.0), SA-Phe- NHOH (m/z calculated value: 387.1 [M+H]+; measured value: 387.0), SA-Pro-NHOH (m/z calculated value: 337.1 [M+H]+; measured value: 337.1). A C18 reversed-phase column (120?, 5 μm, 4.6 × 250 mm; AAPPTec, Louisville, KY, USA) was used, and the product was analyzed by RP-HPLC (Thermo Scientific Spectra System AS300; Thermo-Fisher, Waltham, MA, USA) Product purity was analyzed. The elution conditions were as follows: mobile phase A: 0.1% TFA/water, mobile phase B: 0.1% TFA/acetonitrile, gradient elution: from 10% B to 90% B in 30 min, flow rate: 1.0 mL/min; detection wavelength: UV, 280 or 326 nm. HCA-Phe-NHOH was purified by semi-preparative RP-HPLC columns using an A to B gradient (A: 0.1% TFA aqueous solution, B: 0.1% TFA in acetonitrile; 10% to 90% B for 30 min at a flow rate of 4.0 mL/min) followed by freeze-drying.

82911-69-1
669 suppliers
$7.00/25g

190656-01-0
59 suppliers
$8.00/250mg
Yield:190656-01-0 80%
Reaction Conditions:
with hydroxylamine hydrochloride;sodium hydrogencarbonate in water;ethyl acetate at 5 - 20; for 4 h;
Steps:
Solid phase synthesis of HCA-Phe-NHOH and HCA-Pro-NHOH
Hydroxylamine hydrochloride (834 mg, 12 mmol) was dissolved in 40 mL of aqueous sodium hydrogen carbonate (2.2 g, 26 mmol), and cooled to 5 °C. N-(9-fluorenylmethoxycarbonyloxy) succinimide (Fmoc-OSu, 4.0 g, 12 mmol) dissolved in 40 mL ethyl acetate was added drop wise to the rapidly stirred hydroxylamine solution in an ice-bath and stirred for 4 h at room temperature. The reaction was monitored by TLC (ethyl acetate/hexane = 1:1, Rf = 0.4). After the water layer was removed, the organic layer was washed with saturated aqueous potassium hydrogen sulfate and brine. This organic extract was concentrated in high vacuum, and then N-Fmoc protected hydroxylamine (Fmoc-NHOH) was obtained as a white crystalline solid after trituration in hexane and stored overnight (80% yield). Its structure was identified by 1H NMR (JNM-LA300 spectrometer, JEOL Ltd, Tokyo, Japan): (δH, CDCl3) 4.21 (1H, t, Fmoc CH), 4.32 (2H, d, Fmoc CH2), 7.28-7.43, 7.68, 7.86 (8H, m, Fmoc Ar. CH), 8.77 (1H, s, NH), 9.75 (1H, br s, OH). Fmoc-NHOH (2 equiv) was coupled to 2-chlorotrityl chloride (CTC) resin (1.43 mmol/g) with N,N’-diisopropylethylamine (DIPEA; 4 equiv) in dichloromethane (DCM) for 48 h. Fmoc-NHOH loaded CTC resin was treated with 10% DIPEA/methanol (v/v) to block the remaining chloride groups. The resulting resin was filtered, and its loading level was 1.0 mmol/g, which was determined by Fmoc titration. After treating with 20% piperidine/N-methyl-2-pyrrolidone (NMP) for 30 min to remove Fmoc groups, Fmoc-l-Pro-OH or Fmoc-l-Phe-OH (2 equiv) was coupled to the resin with 2-(1H-7-azabenzotriazol-1-yl)-1,1,3,3,-tetramethyl uronium hexafluorophosphate methanaminium (HATU), 1-hydroxy-7-azabenzotriazole (HOAt), and DIPEA (4 equiv) for 1.5 h at room temperature. After removing Fmoc groups by 20% piperidine/NMP, HCA (2 equiv) was coupled to the amino acid anchored resin with benzotriazole-1-yl-oxy-tris-(dimethylamino)-phosphonium hexafluorophosphate (BOP; 2 equiv), hydroxybenzotriazole (HOBt; 2 equiv) and DIPEA (3 equiv) for 5 h. The final product was cleaved from the resin by 30% trifluoroacetic acid (TFA)/DCM (v/v) for 1 h. The resin was filtered, and the filtrate was concentrated in high vacuum, followed by precipitation with cold diethyl ether. The resulting HCA-Phe-NHOH and HCA-Pro-NHOH were identified by QUATTRO Triple Quardrupole Tandem mass spectrometer (Micromass & Waters, Milford, MA, USA) at National Instrumentation Center for Environmental Management (NICEM): CA-Phe-NHOH (m/z calcd: 343.1 [M+H]+; found: 343.0), CA-Pro-NHOH (m/z calcd: 293.1 [M+H]+; found: 293.1), DHCA-Phe-NHOH (m/z calcd: 345.1 [M+H]+; found: 345.1), DHCA-Pro-NHOH (m/z calcd: 295.1 [M+H]+; found: 295.1), pCoA-Phe-NHOH (m/z calcd: 327.1 [M+H]+; found: 327.1), pCoA-Pro-NHOH (m/z calcd: 277.1 [M+H]+; found: 277.0), FA-Phe-NHOH (m/z calcd: 357.1 [M+H]+; found: 357.1), FA-Pro-NHOH (m/z calcd: 307.1 [M+H]+; found: 307.0), SA-Phe-NHOH (m/z calcd: 387.1 [M+H]+; found: 387.0), SA-Pro-NHOH (m/z calcd: 337.1 [M+H]+; found: 337.1). Their purities were analyzed by RP-HPLC (Thermo Scientific Spectra System AS300; Thermo-Fisher, Waltham, MA, USA) using C18 reverse phase column (120 Å, 5 μm, 4.6 × 250 mm; AAPPTec, Louisville, KY, USA) using the following conditions: gradient elution with A: 0.1% TFA/water, B: 0.1% TFA/acetonitrile; from 10% to 90% over 30 min, a flow rate: 1.0 mL/min; detection: UV, 280 or 326 nm. HCA-Phe-NHOH were purified by a semi-preparative RP-HPLC column using an A to B gradient (A: 0.1% TFA in water, B: 0.1% TFA in acetonitrile; from 10% to 90% B over 30 min, at a flow rate of 4.0 mL/min) and freeze-dried.
References:
Kwak, Seon-Yeong;Yang, Jin-Kyoung;Choi, Hye-Ryung;Park, Kyung-Chan;Kim, Young-Bu;Lee, Yoon-Sik [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 4,p. 1136 - 1142]
28920-43-6
553 suppliers
$5.00/5g

190656-01-0
59 suppliers
$8.00/250mg