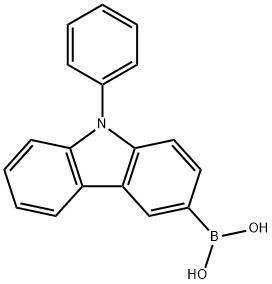
9-Phenyl-9H-carbazol-3-ylboronic acid synthesis
- Product Name:9-Phenyl-9H-carbazol-3-ylboronic acid
- CAS Number:854952-58-2
- Molecular formula:C18H14BNO2
- Molecular Weight:287.12
1153-85-1
325 suppliers
$7.00/1g

121-43-7
382 suppliers
$14.00/25mL

7732-18-5
507 suppliers
$13.50/100ML

854952-58-2
289 suppliers
$18.50/250mg
Yield:854952-58-2 90.2%
Reaction Conditions:
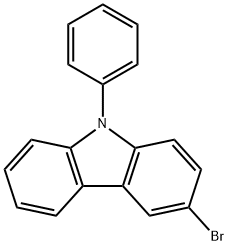
Stage #1: 3-bromo-9-phenyl-9H-carbazolewith n-butyllithium in tetrahydrofuran at -78; for 0.5 h;Inert atmosphere;
Stage #2: Trimethyl borate in tetrahydrofuran; for 0.5 h;Inert atmosphere;
Stage #3: waterwith hydrogenchloride in tetrahydrofuran;Inert atmosphere;
Steps:
1-1
2-neck flask (Two neck flask) in a nitrogen atmosphere gave 1-b '(22.4g, 69.5mmol, 1eq) in anhydrous tetrahydrofuran (THF) fully dissolved in 150ml behind a dry ice / acetone bath (dryice / actone with the bath) it was maintained at a temperature of -78 . Here slowly added an n- butyllithium (nBuLi) (27.8ml, 69.5mmol, 1eq) in 2.5M concentration, the input ends and followed by stirring for 30 minutes, B (OMe) 3 (10.8g, 104.25mmol, 1.5eq) It was added dropwise slowly. Followed by stirring for 30 minutes this was added an excess of 1N aqueous hydrochloric acid solution (HCl solution). And the temperature was elevated gradually to room temperature. Out by using the methane extracted (CHCl3) trichlorosilane, by using the organic layer over magnesium sulfate (MgSO4) to remove water. Ethanol (ethanol) by recrystallization to give the compound 1-c '(18g, yield 90.2%).
References:
KR2016/51654,2016,A Location in patent:Paragraph 0244-0245; 0247

1153-85-1
325 suppliers
$7.00/1g

854952-58-2
289 suppliers
$18.50/250mg

1153-85-1
325 suppliers
$7.00/1g

121-43-7
382 suppliers
$14.00/25mL

854952-58-2
289 suppliers
$18.50/250mg

1153-85-1
325 suppliers
$7.00/1g

5419-55-6
387 suppliers
$12.00/5g

854952-58-2
289 suppliers
$18.50/250mg

121-43-7
382 suppliers
$14.00/25mL
502161-03-7
254 suppliers
$6.00/1g

854952-58-2
289 suppliers
$18.50/250mg