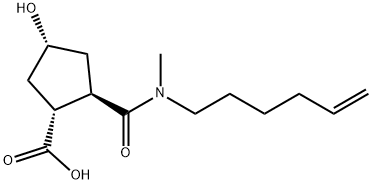
(1R,2R,4R)-2-(hex-5-enyl-methyl-carbamoyl)-4-hydroxy-cyclopentanecarboxylic acid synthesis
- Product Name:(1R,2R,4R)-2-(hex-5-enyl-methyl-carbamoyl)-4-hydroxy-cyclopentanecarboxylic acid
- CAS Number:922727-92-2
- Molecular formula:C14H23NO4
- Molecular Weight:269.34
![(1R,4R,5R)-N-(hex-5-en-1-yl)-N-methyl-3-oxo-2-oxabicyclo[2.2.1]heptane-5-carboxamide](/CAS/20200119/GIF/862174-98-9.gif)
862174-98-9
3 suppliers
inquiry

922727-92-2
11 suppliers
inquiry
Yield:922727-92-2 88%
Reaction Conditions:
Stage #1: (1R,4R,5R)-N-(hex-5-en-1-yl)-N-methyl-3-oxo-2-oxabicyclo[2.2.1]heptane-5-carboxamidewith lithium hydroxide;water at 0; for 1 h;
Stage #2: with hydrogenchloride in water; pH=2 - 3;
Steps:
2.B
A solution of LiOH (105 mg in 4 mL of water) was added at O0C to the lactone amide 23. After Ih, the conversion was completed (HPLC). The mixture was acidified to pH 2-3 with IN HCl, extracted with ethyl acetateAcOEt, dried (MgSO4), evaporated, co-evaporated with toluene several times, and dried under high vacuum overnight to give 520 mg (88%) of the target product 24: m/z = 270 (M+H)+.
References:
WO2007/14921,2007,A1 Location in patent:Page/Page column 77
![2-Oxabicyclo[2.2.1]heptane-5-carboxamide, N-5-hexen-1-yl-N-methyl-3-oxo-](/CAS/20210305/GIF/1027355-52-7.gif)
1027355-52-7
0 suppliers
inquiry

922727-92-2
11 suppliers
inquiry

2695-47-8
294 suppliers
$14.00/5g

922727-92-2
11 suppliers
inquiry
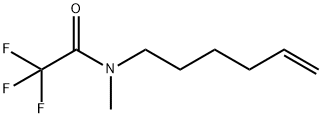
922520-05-6
0 suppliers
inquiry

922727-92-2
11 suppliers
inquiry

55863-02-0
79 suppliers
$32.00/250mg

922727-92-2
11 suppliers
inquiry