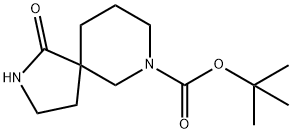
TERT-BUTYL 1-OXO-2,7-DIAZASPIRO[4.5]DECANE-7-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 1-OXO-2,7-DIAZASPIRO[4.5]DECANE-7-CARBOXYLATE
- CAS Number:923009-50-1
- Molecular formula:C13H22N2O3
- Molecular Weight:254.33

24424-99-5
![2,7-DIAZASPIRO[4.5]DECAN-1-ONE HYDROCHLORIDE](/StructureFile/ChemBookStructure20/GIF/CB7815201.gif)
1187173-43-8
![TERT-BUTYL 1-OXO-2,7-DIAZASPIRO[4.5]DECANE-7-CARBOXYLATE](/CAS/GIF/923009-50-1.gif)
923009-50-1
Preparation of Example 44: tert-butyl 1-oxo-2,7-diazaspiro[4.5]decane-7-carboxylate (P44) was synthesized as follows: 2,7-diazaspiro[4.5]decan-1-one hydrochloride (600 mg, 3.15 mmol) was dissolved in 30 mL of dichloromethane (DCM). To this solution, triethylamine (TEA, 1.98 mL, 14.18 mmol) and di-tert-butyl dicarbonate (Boc2O, 895 mg, 4.10 mmol) were added sequentially. The reaction mixture was stirred at room temperature for 4 hours. After completion of the reaction, it was quenched by addition of water and the organic and aqueous layers were separated. The organic layer was washed with aqueous ammonium chloride (NH4Cl), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to remove the solvent to afford the target product tert-butyl 1-oxo-2,7-diazaspiro[4.5]decane-7-carboxylate (P44, 830 mg, quantitative yield) as a colorless oil. Mass spectrum (ESI) m/z: 255.2 [M + H]+.

24424-99-5
868 suppliers
$13.50/25G
![2,7-DIAZASPIRO[4.5]DECAN-1-ONE HYDROCHLORIDE](/StructureFile/ChemBookStructure20/GIF/CB7815201.gif)
1187173-43-8
60 suppliers
$38.00/100mg
![TERT-BUTYL 1-OXO-2,7-DIAZASPIRO[4.5]DECANE-7-CARBOXYLATE](/CAS/GIF/923009-50-1.gif)
923009-50-1
81 suppliers
$106.00/250 mg
Yield: 100%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 4 h;Inert atmosphere;
Steps:
P44 Preparation 44: tert-butyl 1-oxo-2,7-diazaspiro[4,5]decane-7-carboxylate (P44)
Preparation 44: tert-butyl 1-oxo-2,7-diazaspiro[4,5]decane-7-carboxylate (P44)2,7-diazaspiro[4.5]decan-l-one hydrochloride (600 mg, 3.15 mmol), was dissolved in 30 m L of DCM. To this solution, TEA (1.98 mL, 14.18 mmol) and Boc20 (895 mg, 4.10 mmol) were added and the reaction mixture was stirred at RT for 4 hrs. Water was added and the two layers were separated; the organic layer was washed with NH4CI, dried and evaporated to obtain ferf-butyl l-oxo-2,7- diazaspiro[4.5]decane-7-carboxylate (p44, 830 mg, y= quant.), colourless oil.MS (ES) (m/z): 255.2 [M+H]+.
References:
SHIRE INTERNATIONAL GMBH;SEMERARO, Teresa;TARSI, Luca;MICHELI, Fabrizio;LUKER, Tim;CREMONESI, Susanna WO2016/42451, 2016, A1 Location in patent:Page/Page column 84
923009-49-8
13 suppliers
inquiry
![TERT-BUTYL 1-OXO-2,7-DIAZASPIRO[4.5]DECANE-7-CARBOXYLATE](/CAS/GIF/923009-50-1.gif)
923009-50-1
81 suppliers
$106.00/250 mg