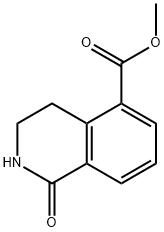
METHYL 1-OXO-1,2,3,4-TETRAHYDROISOQUINOLINE-5-CARBOXYLATE synthesis
- Product Name:METHYL 1-OXO-1,2,3,4-TETRAHYDROISOQUINOLINE-5-CARBOXYLATE
- CAS Number:93258-88-9
- Molecular formula:C11H11NO3
- Molecular Weight:205.21

67-56-1
790 suppliers
$9.00/25ml
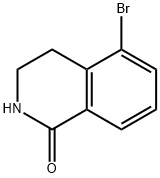
1109230-25-2
83 suppliers
$38.00/100mg
201230-82-2
1 suppliers
inquiry

93258-88-9
23 suppliers
$144.00/100mg
Yield:93258-88-9 80%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;triethylamine in N,N-dimethyl-formamide at 22; under 760.051 Torr; for 156 h;
Steps:
58.58-1 Step 58-1. Synthesis of methyl 1 -oxo- tetrahvdroisoquinoline-5-carboxylate (INT 58-I)
Pd(dppf)Cb DCM (285 mg, 0.35 mmol) was added to a solution of 5-bromo- 3,4-dihydro-2H-isoquinolin-l-one (395 mg, 1.75 mmol) and TEA (1.22 mL, 8.74 mmol) in DMF (6.00 mL) at 22 °C. The mixture was evacuated and refilled with CO for 3 cycles. MeOH (3.08 mL) was added, and the mixture was heated to 85 °C under a CO atmosphere (1 atm) for 16 h. The mixture was diluted with EA (25 mL) and filtered through a pad of Celite. The filtrate was concentrated under reduced pressure. The residue was diluted with EA (100 mL) and LhO (100 mL). The aq. phase was extracted with EA (3 x 25.0 mL). The combined organic layers were washed with brine (50 mL), dried (NaiSO^, filtered, and concentrated. The residue was purified by S1O2 chromatography (hexanes and EA) to provide 285 mg (80%) of methyl l-oxo-l,2,3,4-tetrahydroisoquinoline-5-carboxylate (INT 58-1). LCMS-ESI (m/z) calculated for C11H11NO3: 205.07; found 205.74 (M+H)+, tR = 1.82 min (Method 13). NMR (400 MHz, CDCh) d 8.30 (dd, J = 7.7, 1.5 Hz, 1H), 8.09 (dd, J = 7.8, 1.5 Hz, 1H), 7.42 (dd, J = 7.8 Hz, 1H), 5.95 (s, 1H), 3.92 (s, 3H), 3.58 - 3.52 (m, 2H), 3.49 - 3.40 (m, 2H).
References:
WO2020/198537,2020,A1 Location in patent:Page/Page column 243-245