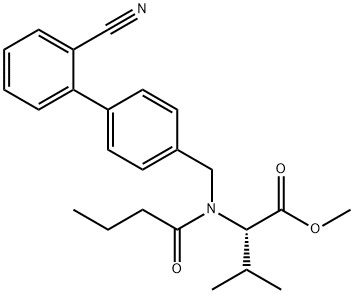
L-Valine, N-[(2'-cyano[1,1'-biphenyl]-4-yl)methyl]-N-(1-oxobutyl)-, methyl ester synthesis
- Product Name:L-Valine, N-[(2'-cyano[1,1'-biphenyl]-4-yl)methyl]-N-(1-oxobutyl)-, methyl ester
- CAS Number:934736-45-5
- Molecular formula:C24H28N2O3
- Molecular Weight:392.49
Yield:934736-45-5 74.9%
Reaction Conditions:
with dmap;triethylamine in dichloromethane at 20 - 45;Inert atmosphere;
Steps:
4.7. N-Butyryl-N-((2'-cyanobiphenyl-4-yl)methyl)-l-Valine methyl ester (6b)
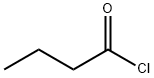
To 31 mL of dichloromethane was added methyl ester 5b (3.97 g, 11.8 mmol), TEA (5 mL, 39.10 mmol) and DMAP (0.09 g, 0.7 mmol). To the mixture was added dropwise carbonyl chloride (2.1 mL, 35.60 mmol) under nitrogen. After stirring for 1 h, the temperature was raised to 45 °C, and the mixture was monitored by TLC till completion. To the mixture was added 10 mL of 1 M dilute aqueous hydrochloride. The organic phase was separated, washed with saturated NaHCO3 solution, and dried over MgSO4. After filtration, the solvent was removed under reduced pressure, and the residue was purified by flash column chromatograph to afford a colorless oil. Yield 74.9%. 1H NMR (CDCl3, 300 MHz) δ: 7.72-7.26 (8H, m, Ph-H), 5.08 (d, J = 15.6 Hz), 5.00 (1H, d, J = 10.5 Hz, -CO-CH-N-), 4.68 (d, J = 17.9 Hz), 4.26 (d, J = 15.6 Hz), 4.04 (2H, d, J = 10.8 Hz, -N-CH2-), 3.43 (s), 3.35 (3H, s, -OCH3), 2.43-2.30 (3H, m, -CO-CH2-, -CHCH3), 1.28-0.84 (9H, m,); 13C NMR (CDCl3, 125 MHz) δ: 178.2, 175.0, 145.1, 141.1, 139.9, 138.3, 137.5, 130.6, 130.6, 130.1, 130.1, 129.2, 129.2, 121.5, 116.4, 66.8, 55.8, 55.4, 39.3, 30.2, 21.1, 20.9, 20.9, 18.6. ESI-MS (m/z): 379.2[M+1]+, 401.2[M+Na]+; HR-MS: 378.1944, C23H26N2O3.
References:
Nie, Yong-Yan;Da, Ya-Jing;Zheng, Hao;Yang, Xiao-Xia;Jia, Lin;Wen, Cai-Hong;Liang, Li-Sha;Tian, Juan;Chen, Zhi-Long [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 8,p. 2747 - 2761] Location in patent:experimental part
72-18-4
814 suppliers
$5.00/25g
![L-Valine, N-[(2'-cyano[1,1'-biphenyl]-4-yl)methyl]-N-(1-oxobutyl)-, methyl ester](/CAS/20210111/GIF/934736-45-5.gif)
934736-45-5
3 suppliers
inquiry
6306-52-1
548 suppliers
$5.00/5g
![L-Valine, N-[(2'-cyano[1,1'-biphenyl]-4-yl)methyl]-N-(1-oxobutyl)-, methyl ester](/CAS/20210111/GIF/934736-45-5.gif)
934736-45-5
3 suppliers
inquiry
![N-[(2'-Cyano[1,1'-biphenyl]-4-yl)methyl]-L-valine methyl ester](/CAS/GIF/137863-89-9.gif)